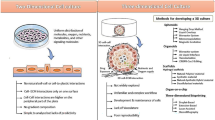
Abstract
Cardiovascular (CV) toxicity is a leading cause of drug attrition and withdrawal. Introducing in vitro assays with higher throughput should permit earlier CV hazard identification and enable medicinal chemists to design-out liabilities. Heretofore, development of in vitro CV assays has been limited by the challenge of replicating integrated cardiovascular physiology while achieving the throughput and consistency required for screening. These challenges appear to be met with a combination of human stem cell-derived cardiomyocytes (CM) which beat spontaneously and monitoring the response with technology that can assess drug-induced changes in voltage dependent contraction such as cellular impedance which has been validated with excellent predictivity for drug-induced arrhythmia and contractility. Here, we review advances in cardiomyocyte impedance with emphasis on stem cell-derived cardiomyocyte models for toxicity screening. Key perspectives include: the electrical principles of impedance technology, impedance detection of cardiomyocyte beating, beat parameter selection/analysis, validation in toxicity and drug discovery, and future directions. As a conclusion, an in vitro screening cascade is proffered using the downstream, inclusive detection of CM impedance assays as a primary screen followed by complementary CM assays chosen to enable mechanism-appropriate follow-up. The combined approach will enhance testing for CV liabilities prior to traditional in vivo models.







Similar content being viewed by others
References
Shah, R. R. (2006). Can pharmacogenetics help rescue drugs withdrawn from the market? Pharmacogenomics, 6, 889–908.
McGuinness, R. P., Proctor, J. M., Gallant, D. L., van Staden, C. J., Ly, J. T., Tang, F. L., et al. (2009). Enhanced selectivity screening of GPCR ligands using a label-free cell based assay technology. Combinational Chemistry and High Throughput Screening, 12(8), 812–823.
Stevens, J. L., & Baker, T. K. (2009). The future of drug safety testing: Expanding the view and narrowing the focus. Drug Discovery Today, 3–4, 162–167.
Ciambrone, G. J., Liu, V. F., Lin, D. C., McGuinness, R. P., Leung, G. K., & Pitchford, S. (2004). Cellular dielectric spectroscopy: A powerful new approach to label-free cellular analysis. Journal of Biomolecular Screening, 6, 467–480.
Giaever, I., & Keese, C. R. (1991). Micromotion of mammalian cells measured electrically. Proceedings of the National Academy of Science, 17, 7896–7900.
Scott, C. W., & Peters, M. F. (2010). Label-free whole-cell assays: Expanding the scope of GPCR screening. Drug Discovery Today, 17–18, 704–716.
Xi, B., Wang, T., Li, N., Ouyang, W., Zhang, W., Wu, J., et al. (2011). Functional cardiotoxicity profiling and screening using the xCELLigence RTCA Cardio System. Journal of the Association for Laboratory Automation, 6, 415–421.
Abassi, Y. A., Xi, B., Li, N., Ouyang, W., Seiler, A., Watzele, M., et al. (2012). Dynamic monitoring of beating periodicity of stem cell-derived cardiomyocytes as a predictive tool for preclinical safety assessment. British Journal of Pharmacology, 5, 1424–1441.
Guo, L., Abrams, R. M., Babiarz, J. E., Cohen, J. D., Kameoka, S., Sanders, M. J., et al. (2011). Estimating the risk of drug-induced proarrhythmia using human induced pluripotent stem cell-derived cardiomyocytes. Toxicological Sciences, 1, 281–289.
Parsons, J. T., Horwitz, A. R., & Schwartz, M. A. (2010). Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nature Reviews Molecular Cell Biology, 9, 633–643.
Layland, J., & Kentish, J. C. (1999). Positive force- and [Ca2+]i-frequency relationships in rat ventricular trabeculae at physiological frequencies. American Journal of Physiology, 1(Pt 2), H9–H18.
Lieu, D. K., Liu, J., Siu, C. W., McNerney, G. P., Tse, H. F., Abu-Khalil, A., et al. (2009). Absence of transverse tubules contributes to non-uniform Ca(2+) wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes. Stem Cells and Development, 10, 1493–1500.
Delcarpio, J. B., Claycomb, W. C., & Moses, R. L. (1989). Ultrastructural morphometric analysis of cultured neonatal and adult rat ventricular cardiac muscle cells. American Journal of Anatomy, 4, 335–345.
Germanguz, I., Sedan, O., Zeevi-Levin, N., Shtrichman, R., Barak, E., Ziskind, A., et al. (2011). Molecular characterization and functional properties of cardiomyocytes derived from human inducible pluripotent stem cells. Journal of Cellular and Molecular Medicine, 1, 38–51.
Dibb, K. M., Eisner, D. A., & Trafford, A. W. (2007). Regulation of systolic [Ca2+]i and cellular Ca2+ flux balance in rat ventricular myocytes by SR Ca2+ L-type Ca2+ current and diastolic [Ca2+]i. The Journal of Physiology, 2, 579–592.
Korhonen, T., Hanninen, S. L., & Tavi, P. (2009). Model of excitation-contraction coupling of rat neonatal ventricular myocytes. Biophysical Journal, 3, 1189–1209.
Peters, M. F., Scott, C. W., Ochalski, R., & Dragan, Y. P. (2012). Evaluation of cellular impedance measures of cardiomyocyte cultures for drug screening applications. Assay and Drug Development Technologies, 6, 525–532.
Kepp, O., Galluzzi, L., Lipinski, M., Yuan, J., & Kroemer, G. (2011). Cell death assays for drug discovery. Nature Reviews Drug Discovery, 3, 221–237.
Lamore, S. D., Kamendi, H. W., Scott, C. W., Dragan, Y. P., & Peters, M. F. (2013). Cellular impedance assays for predictive preclinical drug screening of kinase inhibitor cardiovascular toxicity. Toxicological Sciences, 2, 402–413.
Sirenko, O., Crittenden, C., Callamaras, N., Hesley, J., Chen, Y. W., Funes, C., et al. (2013). Multiparameter in vitro assessment of compound effects on cardiomyocyte physiology using iPSC cells. Journal of Biomolecular Screening, 1, 39–53.
Schiller, L. R., & Johnson, D. A. (2008). Balancing drug risk and benefit: Toward refining the process of FDA decisions affecting patient care. American Journal of Gastroenterology, 4, 815–819.
Redfern, W. S., Carlsson, L., Davis, A. S., Lynch, W. G., MacKenzie, I., Palethorpe, S., et al. (2003). Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: Evidence for a provisional safety margin in drug development. Cardiovascular Research, 1, 32–45.
Lu, H. R., Vlaminckx, E., Hermans, A. N., Rohrbacher, J., Van Ammel, K., Towart, R., et al. (2008). Predicting drug-induced changes in QT interval and arrhythmias: QT-shortening drugs point to gaps in the ICHS7B Guidelines. British Journal of Pharmacology, 7, 1427–1438.
Guo, L., Coyle, L., Abrams, R. M., Kemper, R., Chiao, E. T., & Kolaja, K. L. (2013). Refining the human iPSC-cardiomyocyte arrhythmic risk assessment model. Toxicological Sciences, 2, 581–594.
Gintant, G. (2011). An evaluation of hERG current assay performance: Translating preclinical safety studies to clinical QT prolongation. Pharmacology & Therapeutics, 2, 109–119.
Sager, P. T., Gintant, G., Turner, J. R., Pettit, S., & Stockbridge, N. (2014). Rechanneling the cardiac proarrhythmia safety paradigm: A meeting report from the cardiac safety research consortium. American Heart Journal, 3, 292–300.
Jonsson, M. K., Wang, Q. D., & Becker, B. (2011). Impedance-based detection of beating rhythm and proarrhythmic effects of compounds on stem cell-derived cardiomyocytes. Assay and Drug Development Technologies, 6, 589–599.
Nguemo, F., Saric, T., Pfannkuche, K., Watzele, M., Reppel, M., & Hescheler, J. (2012). In vitro model for assessing arrhythmogenic properties of drugs based on high-resolution impedance measurements. Cellular Physiology and Biochemistry, 5–6, 819–832.
Chi, K. R. (2013). Regulatory watch: Speedy validation sought for new cardiotoxicity testing strategy. Nature Reviews Drug Discovery, 9, 655.
Mellor, H. R., Bell, A. R., Valentin, J. P., & Roberts, R. R. (2011). Cardiotoxicity associated with targeting kinase pathways in cancer. Toxicological Sciences, 1, 14–32.
Harmer, A. R., Abi-Gerges, N., Morton, M. J., Pullen, G. F., Valentin, J. P., & Pollard, C. E. (2012). Validation of an in vitro contractility assay using canine ventricular myocytes. Toxicology and Applied Pharmacology, 2, 162–172.
Force, T., & Kolaja, K. L. (2011). Cardiotoxicity of kinase inhibitors: The prediction and translation of preclinical models to clinical outcomes. Nature Reviews Drug Discovery, 2, 111–126.
Doherty, K. R., Wappel, R. L., Talbert, D. R., Trusk, P. B., Moran, D. M., Kramer, J. W., et al. (2013). Multi-parameter in vitro toxicity testing of crizotinib, sunitinib, erlotinib, and nilotinib in human cardiomyocytes. Toxicology and Applied Pharmacology, 1, 245–255.
Cohen, J. D., Babiarz, J. E., Abrams, R. M., Guo, L., Kameoka, S., Chiao, E., et al. (2011). Use of human stem cell derived cardiomyocytes to examine sunitinib mediated cardiotoxicity and electrophysiological alterations. Toxicology and Applied Pharmacology, 1, 74–83.
Lim, J., Taoka, B. M., Lee, S., Northrup, A., Altman, M., Sloman, D., et al. (2011). Pyrazolo[1,5-A]pyrimidines as MARK inhibitors. World Patent WO 2011/087999 A1, 21 July 2011.
Anastassiadis, T., Deacon, S. W., Devarajan, K., Ma, H., & Peterson, J. R. (2011). Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nature Biotechnology, 11, 1039–1045.
Davis, M. I., Hunt, J. P., Herrgard, S., Ciceri, P., Wodicka, L. M., Pallares, G., et al. (2011). Comprehensive analysis of kinase inhibitor selectivity. Nature Biotechnology, 11, 1046–1051.
Carlson, C., Koonce, C., Aoyama, N., Einhorn, S., Fiene, S., Thompson, A., et al. (2013). Phenotypic screening with human iPS cell-derived cardiomyocytes: HTS-compatible assays for interrogating cardiac hypertrophy. Journal of Biomolecular Screening, 10, 1203–1211.
Satoh, H., Delbridge, L. M., Blatter, L. A., & Bers, D. M. (1996). Surface:volume relationship in cardiac myocytes studied with confocal microscopy and membrane capacitance measurements: Species-dependence and developmental effects. Biophysical Journal, 3, 1494–1504.
Feinberg, A. W., Alford, P. W., Jin, H., Ripplinger, C. M., Werdich, A. A., Sheehy, S. P., et al. (2012). Controlling the contractile strength of engineered cardiac muscle by hierarchal tissue architecture. Biomaterials, 23, 5732–5741.
Blazeski, A., Zhu, R., Hunter, D. W., Weinberg, S. H., Zambidis, E. T., & Tung, L. (2012). Cardiomyocytes derived from human induced pluripotent stem cells as models for normal and diseased cardiac electrophysiology and contractility. Progress in Biophysics and Molecular Biology, 2–3, 166–177.
Uppal H. (2013). Harnessing stem cells for predictive toxicology: Meeting the challenges of drug discovery today. http://webinar.sciencemag.org/webinar/archive/harnessing-stem-cells-predictive-toxicology#speaker-bio-467. Accessed July 17, 2013.
Laverty, H., Benson, C., Cartwright, E., Cross, M., Garland, C., Hammond, T., et al. (2011). How can we improve our understanding of cardiovascular safety liabilities to develop safer medicines? British Journal of Pharmacology, 4, 675–693.
Liang, P., Lan, F., Lee, A. S., Gong, T., Sanchez-Freire, V., Wang, Y., et al. (2013). Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation, 16, 1677–1691.
Harris, K., Aylott, M., Cui, Y., Louttit, J. B., McMahon, N. C., & Sridhar, A. (2013). Comparison of electrophysiological data from human-induced pluripotent stem cell-derived cardiomyocytes to functional preclinical safety assays. Toxicological Sciences, 2, 412–426.
Navarrete, E. G., Liang, P., Lan, F., Sanchez-Freire, V., Simmons, C., Gong, T., et al. (2013). Screening drug-induced arrhythmia events using human induced pluripotent stem cell-derived cardiomyocytes and low-impedance microelectrode arrays. Circulation, 11(Suppl 1), S3–S13.
Sirenko, O., Cromwell, E. F., Crittenden, C., Wignall, J. A., Wright, F. A., & Rusyn, I. (2013). Assessment of beating parameters in human induced pluripotent stem cells enables quantitative in vitro screening for cardiotoxicity. Toxicology and Applied Pharmacology, 3, 500–507.
Cerignoli, F., Charlot, D., Whittaker, R., Ingermanson, R., Gehalot, P., Savchenko, A., et al. (2012). High throughput measurement of Ca(2)(+) dynamics for drug risk assessment in human stem cell-derived cardiomyocytes by kinetic image cytometry. Journal of Pharmacological and Toxicological Methods, 3, 246–256.
Alford, P. W., Feinberg, A. W., Sheehy, S. P., & Parker, K. K. (2010). Biohybrid thin films for measuring contractility in engineered cardiovascular muscle. Biomaterials, 13, 3613–3621.
Pointon, A., Abi-Gerges, N., Cross, M. J., & Sidaway, J. E. (2013). Phenotypic profiling of structural cardiotoxins in vitro reveals dependency on multiple mechanisms of toxicity. Toxicological Sciences, 2, 317–326.
Bers, D. M. (2002). Cardiac excitation-contraction coupling. Nature, 6868, 198–205.
Scott, C. W., Peters, M. F., & Dragan, Y. P. (2013). Human induced pluripotent stem cells and their use in drug discovery for toxicity testing. Toxicology Letters, 1, 49–58.
Acknowledgments
We thank Charlie Keese and Xiaobo Wang for comments on Sect. 2 and Amy Pointon for calcium flux data in Fig. 2. This work has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peters, M.F., Lamore, S.D., Guo, L. et al. Human Stem Cell-Derived Cardiomyocytes in Cellular Impedance Assays: Bringing Cardiotoxicity Screening to the Front Line. Cardiovasc Toxicol 15, 127–139 (2015). https://doi.org/10.1007/s12012-014-9268-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-014-9268-9