Abstract
Mitochondria serve as the primary site for metabolizing the three major nutrients, underscoring their pivotal role in cellular energy metabolism and the regulation of signaling pathways. Mitochondrial homeostatic imbalance is a key pathological cause of the development of many diseases. Hence, preserving mitochondrial homeostasis is vital for the normal growth and development of cells and organisms. Living organisms have evolved intricate regulatory mechanisms to ensure cellular mitochondrial homeostasis. This review focuses on recent advancements in comprehending the mechanisms responsible for maintaining mitochondrial homeostasis and addresses the current challenges in this field. We also provide an overview of the key functions of mitochondria in both physiological and pathological conditions. Emphasizing the potential therapeutic implications, we discuss strategies for preserving mitochondrial homeostasis, recognizing its significance in mitigating various health conditions.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Homeostasis is a dynamic self-regulating equilibrium that enables an organism to maintain systemic stability and conduct normal life activities (Wang 2022). When this dedicated balance is disrupted and the regulatory systems governing homeostasis are perturbed, the organism undergoes a series of functional, structural, and metabolic changes that can lead to diseases or even mortality (Wang 2022). Therefore, homeostatic medicine, which focuses on treating disruptions to homeostasis and dynamically restoring the equilibrium of various systems, holds immense significance for health maintenance as well as the diagnosis and treatment of diseases. Intracellular homeostasis serves as the fundamental basis for tissue and organ stability, fostering a conducive environment for the exchange of information and real-time responses among organs. This, in turn, establishes a stable physiological and metabolic microenvironment within the body. Therefore, the preservation of cellular homeostasis emerges as the cornerstone of life, encompassing a multitude of regulatory mechanisms such as intracellular molecular interactions, metabolic homeostasis, and the maintenance of energy balance.
In recent years, it has become evident that the proper performance of mitochondria, the energy center of all eukaryotic cells, is integral to maintaining cellular homeostasis (Wang 2022; Wong et al. 2019). Mitochondria not only act as a "powerhouse" to synthesize adenosine triphosphate (ATP) through oxidative phosphorylation, but also as an "information processor" that plays a central role in Ca2+storage, initiation of cell differentiation and apoptosis, and synthesis of biomolecules, including plasma compounds, neurotransmitters, and hormones (Picard and Shirihai 2022). Thus, there is a strong link between mitochondrial dysfunction and the pathogenesis of a wide range of human diseases involving multiple systems, encompassing neurological disorders, cardiovascular diseases, liver diseases, renal dysfunction, diabetes mellitus, and malignancies associated with DNA damage response (Harrington et al. 2023). In addition, mitochondria are semi-autonomous organelles with their own genetic material and systems, capable of independent mitochondrial DNA replication, transcription, and translation (Wang et al. 2021). However, mitochondrial DNA is more susceptible to oxidative damage and mutations than nuclear DNA because of its proximity to the electron transport system, which generates reactive oxygen species and is unable to synthesize glutathione to scavenge peroxides (Huang 2020). Owing to their extensive involvement in numerous cellular biological processes, such as cell fate determination and senescence, mitochondria require fine-grained, multilayered quality control systems to ensure their normal function, thereby upholding cellular and organismal homeostasis.
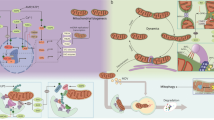
In this review, we provide a comprehensive overview of the mechanisms that regulate cellular mitochondrial homeostasis and emphasize their interplays, which mainly include mitochondrial metabolism, quality control, inter-organelle contact, as well as communication with the nucleus. We also explore the potential targets of cellular mitochondrial homeostasis in disease therapy and describe the limitations and future directions of these innovative targets (Fig. 1).
Mechanisms of mitochondrial homeostasis. Mitochondrial metabolism, quality control mechanisms, mitochondria-organelle interaction network, and mitochondria-nucleus communication collectively play indispensable roles in maintaining mitochondrial operation. These integrated processes collaborate to uphold mitochondrial homeostasis, thereby ensuring cellular and organismal homeostasis. Created with Biorender
2 Regulation of mitochondrial metabolism
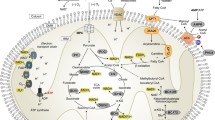
Mitochondrial metabolism plays a central role in the intricate cellular metabolic network due to processes such as the tricarboxylic acid (TCA) cycle, oxidative phosphorylation (OXPHOS), fatty acid oxidation (FAO), nucleotide synthesis, and amino acid, which all take place within mitochondria (Anderson et al. 2019; Spinelli and Haigis 2018). In addition to serving as an energy source, mitochondrial metabolites also function as mediators of mitochondrial signaling to influence cell cycle progression (Liu and Birsoy 2023). Mitochondrial energy metabolism and reduction–oxidation (redox) signaling interact and complement each other to maintain mitochondrial homeostasis. In normal epithelial cells and endothelial cells, extracellular matrix (ECM) detachment will destroy cell metabolic activity, increase the production of mitochondrial reactive oxygen species (ROS), enhance oxidative stress, and then cause anoikis (Fiocca 1988). However, this process can be delayed by restricting mitochondrial respiration, which can limit the flux of glucose through the TCA cycle through already reduced nutrient uptake and negative regulation of pyruvate dehydrogenase (PDH) activity (Kamarajugadda 2012; Grassian et al. 2011). Untransformed epithelial cells phosphorylate and inhibit PDH by up-regulating expression of pyruvate dehydrogenase kinase 4 (PDHK4) after matrix detachment, thereby limiting glucose flow in the TCA cycle (Kamarajugadda 2012; Grassian et al. 2011). Accordingly, mitochondrial metabolism can be regulated through a redox signaling process that stimulates endogenous H2O2 production and leads to impaired ATP production in mitochondria. The γ subunit of the TCA circulating enzyme isocitrate dehydrogenase 3 (IDH3γ) is a redox switch that links its modification to alterations in mitochondrial metabolism (Nanadikar 2023). Dysregulation of mitochondrial energy and redox signaling can lead to the disruption of mitochondrial homeostasis and promote the development, progression, and evasion of various diseases, including cancer and immune disorders (Aranda-Rivera 2021; Schirrmacher 2020) (Fig. 2).
Regulation of mitochondrial metabolism. Mitochondrial metabolism is a complex cellular metabolic network because processes such as the tricarboxylic acid cycle, oxidative phosphorylation, fatty acid oxidation, etc. all occur within mitochondria. In addition to serving as an energy source, mitochondrial metabolites like reactive oxygen species also act as mediators of mitochondrial signaling to influence cell cycle progression. Balance of both energy metabolism and redox signaling is crucial for the maintenance of mitochondrial metabolism. ATP adenosine triphosphate; ADP adenosine diphosphate; DHAP, dihydroxyacetone phosphate; G3P, glycerol-3-phosphate; TCA, tricarboxylic acid; α-KG, α-ketoglutarate; Succinyl-CoA, succinyl coenzyme A; Acetyl-CoA, acetyl coenzyme A; GSH, glutathione; GSSG, glutathione (oxidized); NADPH, the reduced form of nicotinamide adenine dinucleotide phosphate; NADP+ , nicotinamide adenine dinucleotide phosphate. Created with Biorender
2.1 Energy metabolism
ATP is mainly involved in intracellular substance transport and metabolism, during cell growth and metabolic activities. ATP can act as an important cofactor to participate in a large number of intracellular enzyme-catalyzed reactions, linking substance metabolic pathways in series or in parallel to form a complex network system (Ruccolo 2022). Intracellular energy status also affects the rate of cell growth and metabolic reactions; therefore, ATP has an important influence on the anabolism of proteins, lipids, nucleotides, and amino acids (Zhu and Thompson 2019). ATP also plays a key role as a signaling molecule in post-transcriptional modification and signal transduction systems (Vultaggio-Poma 2020).
Elevated mitochondrial oxidative metabolism is a well-established metabolic hallmark of leukemia (Chen et al. 2020). Leukemia cells exhibit heterogeneity in energy and substance metabolism. In acute myeloid and lymphoblastic leukemia, the inhibition of mitochondrial respiration disarms resistance to chemotherapeutic agents (Carter 2020). The phosphatidylinositol 4-kinase alpha (PI4KA) inhibitor cefadroxil (CEP) destabilizes the PI4KA/tetratricopeptide repeat domain 7 (TTC7)/family with sequence similarity 126 (FAM126) complex and inhibits ATP production by modulating the extracellular signal-regulated kinase (ERK)/adenosine monophosphate-activated protein kinase (AMPK)/OXPHOS axis, thus enhancing chemotherapeutic drug resistance in drug-resistant leukemia cells in vitro and in vivo, thereby providing a new therapeutic strategy for the treatment of refractory leukemia (Jiang 2022). In addition, the alteration of glucose metabolic flux from mitochondrial OXPHOS to aerobic glycolysis is considered to be one of the essential features of cancer cells (Martinez-Reyes and Chandel 2021). In this regard, mammalian target of rapamycin (mTOR)-mediated M2 isoform of pyruvate kinase (PKM2), a rate-limiting enzyme in glycolysis, interacts with mitofusin 2 (MFN2), a key regulator of mitochondrial fusion, to promote mitochondrial fusion, OXPHOS, and ATP production, while inhibiting glycolysis, thus playing a key role in regulating cancer cell proliferation (Li 2019). Moreover, under cold conditions and stimulation by β-adrenergic receptors, white adipose tissue can transform into beige adipocytes rich in uncoupling protein 1 (UCP1) (Razzoli et al. 2018). UCP1 eliminates the proton gradient across the inner mitochondrial membrane, uncouples the respiratory chain via OXPHOS, reduces ATP synthesis, and releases energy in the form of heat (Cohen and Kajimura 2021). Recent studies have shown that white adipose tissue induces local thermogenesis through heat shock transcription factor 1(HSF1) activation and promotes browning, resulting in significant heat production and energy expenditure (Li 2022). These findings demonstrate that thermal therapy can prevent and treat obesity, while improving metabolic disorders through the regulation of mitochondrial energy metabolism homeostasis, providing new insights for addressing obesity and metabolic disorders (Li 2022). Moreover, mitochondrial dysfunction is thought to be one of the causes of type 2 diabetes mellitus (T2DM). Studies have found that after the specific knockout of TFAM in mouse islet beta cells, ATP production was reduced, insulin secretion was decreased, and mice showed T2DM symptoms. Furthermore, energy metabolism is closely associated with aging. Cytoplasmic polyadenylation element binding protein 4 (CPEB4), an RNA-binding protein, may target impaired mitochondrial energy metabolism in senescent muscle stem cells by controlling mitochondrial translation to improve the function of senescent muscle stem cells, thus providing new theoretical evidence for the possibility of controlling cellular senescence by regulating mitochondrial energy metabolism (Zeng 2023).
In conclusion, mitochondrial energy metabolism is a complex and important field that requires in-depth research in several aspects in the future. By delving into the regulatory mechanisms of mitochondrial energy metabolism (focusing on the maintenance of mitochondrial homeostasis), its relationship with cellular functions (the interplay between cell growth, differentiation, and apoptosis) and human health (its role in the onset and progression of human diseases), and its engineering applications (to better utilize the mechanisms of mitochondrial energy metabolism), we can better make use of mitochondrial functions and provide new insights into future disease treatments and health management by providing new ideas and approaches.
2.2 Redox signaling
In organisms, redox metabolism and the maintenance of its homeostasis is one of the most fundamental cellular activities of life. In the electron transport chain (ETC), about 2% of O2binds to electrons that are not transmitted to terminal oxidases but "leak" out of the respiratory chain, resulting in the production of ROS, which is also the predominant oxidant within the mitochondrial redox signaling (Handy and Loscalzo 2012). Among them, nuclear respiratory factors (NRF)1 and NRF2 regulate each other and send multilevel signaling integration and local fine-tuning to the gene expression network through the function of their transcription factors, thus maintaining cellular and mitochondrial redox homeostasis and energy metabolism homeostasis (Cui 2021; Esteras and Abramov 2022). Recent studies have found that the deletion of NRF1 led to a substantial increase in ROS levels and indicators of oxidative stress (including lipid peroxidation) in cells. Although NRF2 was significantly upregulated, the increase in ROS and oxidative damage persisted, suggesting NRF1 knockdown did not prevent a significant increase in intracellular and mitochondrial ROS. Furthermore, when NRF2 was knocked down, ROS levels increased significantly; however, no compensatory changes in NRF1 expression were observed (Hu 2022). Thus, when redox signaling is altered beyond the physiological threshold, mitochondrial homeostasis is disrupted, resulting in the inability of cells to respond adaptively to changes in the internal and external environments, which leads to the development of potential diseases, such as inflammatory aging and tumors.
Upon sustained antioxidant signaling or mitochondrial inactivation, CUL2FEM1B and folliculin interacting protein 1 (FNIP1) form a ubiquitin-dependent stress combination that regulates mitochondrial ROS production and maintains redox signaling homeostasis during myoblast metabolism by degrading or controlling FNIP1 (Manford 2020). Mitochondrial dysfunction may contribute to rheumatoid arthritis by modulating innate immunity through redox-sensitive inflammatory pathways or by directly activating inflammasomes. Suppression of the ROS-nuclear factor (NF)-κB-nucleotide-binding domain leucine-rich repeat-containing family pyrin domain containing 3 (NLRP3) axis has demonstrated efficacy in alleviating symptoms and attenuating inflammation associated with rheumatoid arthritis, providing evidence for targeting mitochondrial redox signaling against inflammation (Jing 2021). Furthermore, metastatic breast cancer cells preferentially metastasize to organs characterized by a soft microenvironment and a pliable extracellular matrix, which facilitates the maintenance of redox signaling homeostasis (Gensbittel 2021). Paradoxically, this compensatory mechanism increases resistance to oxidative stress and to chemotherapeutic drugs that rely on ROS. Targeting the key oxidative stress factors Dynamin-related protein 1 (DRP1) and NRF2, in thoracic cancer cells can restore cisplatin sensitivity while preventing the reactivation of metastasized cancer cells (Romani 2022). A suitable redox environment is essential for normal neuronal function. The increase in lactate intake by neurons leads to the enhancement of mitochondrial energy metabolism and the production of more ROS, resulting in oxidative stress in neurons. Even worse, damage to mitochondria and ATP synthesis produces more reactive oxygen species. This vicious cycle eventually leads to axonal degeneration in the peripheral nervous system (Jia 2021). Moreover, in proliferating and differentiated neuroblastoma Neuro 2a (N2a) cells, sulforaphane-mediated NRF2 activation leads to reductive stress that blocks neurogenesis through glycogen synthase kinase 3 beta(GSK3β)/tubulin associated unit (TAU) signaling and protein toxicity (KK 2020). If the ROS concentration easily reaches a threshold, it causes oxidative damage and leads to aging. A study has utilized Caenorhabditis elegans which has been used as a model organism to elucidate and quantify the threshold of ROS by examining the effects of varying concentrations of paraquat-induced ROS on lifespan. Its findings suggest that various stimuli early in life, such as starvation, exercise, and heat exposure, can enhance the redox stress responsiveness of cells and individuals, thereby promoting healthy aging (Miranda-Vizuete and Veal 2017). Therefore, optimizing the plasticity of mitochondrial redox ability may have significant implications for maintaining a stable state of mitochondrial redox signaling.
ROS has long been recognized as an undesirable byproduct of mitochondrial metabolism. Elevated levels of ROS may lead to nonspecific oxidation of targets, resulting in damage to macromolecules and impairing their function, while also triggering stress response mechanisms, such as inflammation, tumor growth, nerve injury, and impaired stem cell differentiation. However, it is now increasingly acknowledged that ROS are beneficial to cells in most states and serve as important signaling molecules in addition to their involvement in maintaining the mitochondrial redox signaling balance. In the future, the generation and regulation mechanisms of ROS signaling in mitochondria (localization and regulation of ROS-generating sites), the interactions between ROS signaling and cellular signaling, and the interactions between ROS signaling and other organelles should be thoroughly investigated. It is necessary to consider the biphasic and dose-dependent roles of ROS, as well as the different functions of mitochondrial ROS in different cell types, to provide the basis for a more precise targeted disease therapy by providing new strategies.
3 Mitochondrial quality control
Mitochondrial quality control (MQC) is an integrated network for monitoring mitochondrial quality and is an endogenous cellular protective program that is critical for maintaining mitochondrial homeostasis and function (Ni et al. 2015). MQC co-regulates the maintenance of mitochondrial homeostasis by orchestrating a wide range of processes such as biogenesis, mitochondrial autophagic degradation, mitochondrial fission, fusion, and mitochondrial proteostasis (Roca-Portoles and Tait 2021). At the cellular level, mitochondrial biogenesis and autophagy facilitate mitochondrial renewal by eliminating damaged mitochondria and synthesizing healthy replacements. At the organelle level, mitochondria dynamically alter their shape, size, and position through division and fusion events to maximize their utilization efficiency. From a molecular perspective, mitochondria maintain protein homeostasis and perform normal physiological functions via proteolysis in response to oxidative stress, misfolding events, impaired proteins, or damage arising from defects in the ETC. Overall, mitochondrial quality control ensures normal mitochondrial quantity, quality, and function through a variety of biological processes and signaling regulatory mechanisms (Fig. 3).
Mitochondrial quality control. Mitochondrial quality control ensures normal mitochondrial quantity, quality, and function through a variety of biological processes and signaling regulatory mechanisms, including biogenesis and mitophagy, fission and fusion, as well as mitochondrial proteostasis. Mfn1/2, mitochondrial fusion protein 1/2; Opa 1, optic atrophy 1; Drp-1, dynamin-related protein 1; PGC1α, peroxisome proliferator-activated receptor γ coactivator 1-alpha; LC3II, microtubule-associated proteins light chain 3 II; FUNDC1, FUN14 domain-containing 1; PINK1; PTEN-induced kinase 1; TOM, translocator of the outer mitochondrial membrane; TIM, translocator of the inner mitochondrial membrane; UPRmt, mitochondrial unfolded protein response. Created with Biorender
3.1 Mitochondrial biogenesis and mitophagy
Mitochondrial mass and quantity are precisely regulated by two fundamental and opposing mechanisms, mitochondrial biogenesis and mitophagy, in response to the cell's energy demands and other cellular and environmental cues. There is close coordination between mitochondrial biogenesis and mitophagy, and impairment of either process can cause an imbalance in mitochondrial homeostasis, which in turn leads to disease.
Mitochondrial biogenesis is a regenerative program that maintains mitochondrial populations by replacing old and damaged mitochondria with new and healthy ones and is co-regulated by a variety of transcription factors in both mitochondrial DNA (mtDNA) and nuclear DNA (nDNA) (Zhou et al. 2021). The generation of new mitochondria through mitochondrial biogenesis promotes ATP production to meet the metabolic demands in physiological and pathological states. Current theories concur that the peroxisome proliferator-activated receptor gamma (PPARG) coactivator (CoA) 1 alpha (PGC1α)/NRFs pathway serves as the pivotal link in governing mitochondrial biosynthesis regulation (Ventura-Clapier et al. 2008). Zinc finger 281 (ZNF281), a member of the ZNF family of transcription factors, negatively regulates mitochondrial biogenesis in hepatocytes by targeting the NRF1/PGC1α- mitochondrial transcription factor A (TFAM) axis and is a potential therapeutic agent for hepatocellular carcinoma (Zhao 2023). Moreover, other molecules such as mitochondrial metabolites are involved in the regulation of biogenesis. Fumarate is the product of succinate which is catalyzed by succinate dehydrogenase (SDH) in the tricarboxylic acid cycle, called "carcinogenic metabolite" due to its promotion of cancer development (Jardim-Messeder 2017). Studies have shown that fumarate upregulates mitochondrial DNA levels in multiple solid tumor cell lines through a mechanism dependent on the mitochondrial metabolic enzyme malic enzyme (ME2). These findings highlight that the suppression of cancer cell mitochondrial biogenesis can exert control over the initiation and progression of cancer (Wang 2021). Neurodegenerative diseases, such as Parkinson's disease and Alzheimer's disease, are also strongly associated with dysfunctional mitochondrial biogenesis. Parkin protects midbrain neurons from neuroinflammation and degeneration by regulating mitochondrial autophagy, mitochondrial biogenesis, and mtDNA maintenance pathways (Wasner 2022). Transferring mitochondria from stem cells to targeting cells is a novel approach to target mitochondrial biosynthesis. Transplantation of mitochondria derived from bone marrow stromal cells (BMSCs) was found to ameliorate knee osteoarthritis by enhancing chondrocyte mitochondrial function and promoting mitochondrial biogenesis through activation of PGC1α signaling (Yu et al. 2022).
Mitophagy, on the other hand, is selective autophagy that maintains mitochondrial functional integrity and cellular homeostasis by specifically removing dysfunctional mitochondria from the cytoplasm. The mechanisms of mitophagy can be broadly categorized into ubiquitin-dependent and non-ubiquitin-dependent pathways. The former is predominantly dependent on the phosphatase and tensin homolog (PTEN) induced kinase 1 (PINK1)/Parkin pathway (Eiyama and Okamoto 2015). In normal mitochondria, PINK1 is constantly translocated to the inner mitochondrial membrane, followed by clearance. However, when the mitochondrial membrane potential is impaired, the entry of PINK1 into the inner mitochondrial membrane is obstructed, leading to the stable accumulation of PINK1 in the outer membrane and initiation of mitophagy through a cascade of reactions (Wang 2020). Unlike ubiquitin-dependent mitophagy, non-ubiquitin-dependent mitophagy relies primarily on mitophagy receptors containing microtubule-associated protein 1 light chain 3 (LC3) interaction domains that directly bind to LC3 to initiate mitophagy (Li 2022). Studies have shown that Rosi (rosiglitazone) can affect the transcription of PINK1 by inhibiting inflammation and reducing forkhead box O1 (FOXO1) expression, thereby inhibiting mitophagy and promoting neural stem cell (NSC) proliferation, enhancing motor function recovery in rats with spinal cord injury (Meng 2022). However, stem cell therapy prevents aging by stimulating mitophagy. Anti-aging therapy using adipose mesenchymal stem cells interaction with senescent cells and animal models of premature aging has been shown to accelerate mitophagy, thereby eliminating intracellular ROS and improving mitochondrial quality (Lv 2021). In addition, a lack of mitophagy can lead to kidney disease. PINK1 or PARK2-mediated mitophagy loss significantly increases the production of mitochondrial reactive oxygen species (mROS) and amplifies the transforming growth factor (TGF)-β1/Smad2/3 cascade, thereby accelerating renal fibrosis (Li 2020). Meanwhile, mitophagy inducer significantly delayed renal fibrosis in mice (Jin 2022).
Similarly, the precise regulation of the coordination between mitochondrial biogenesis and mitophagy is essential for maintaining optimal mitochondrial content and function. Activation of FUN14 domain containing 1 (FUNDC1)-mediated mitophagy in brown adipose tissue (BAT) under cold conditions. However, when FUNDC1 is specifically absent from BAT, it significantly inhibits cold-induced mitochondrial biogenesis, leading to the accumulation of damaged mitochondria, mitochondrial dysfunction, and reduced tolerance to cold temperatures (Liu 2021). This mechanism facilitates mitochondrial turnover and maintains functional mitochondrial networks by simultaneously activating the two opposing processes. Meanwhile, there was an upsurge in mitophagy during the initial stage of differentiation of induced pluripotent stem cells into vascular endothelial cells (Krantz 2021). The expression of PPARGC1A/PGC1α, a regulator involved in mitochondrial biogenesis, was also significantly increased with further cell differentiation, which may be related to the cleavage of PGAM5 during differentiation and the enhancement of the transcriptional activity of β-catenin 1 (CTNNB1) in endothelial progenitor cells (Gajwani and Rehman 2022). This finding supports the interaction between mitophagy and mitochondrial compensatory reprogramming during stem cell differentiation. Furthermore, deletion of Park2 (encoding Parkin) in white adipocytes enhances mitochondrial biogenesis through increasing the stability of PGC1α protein with activation of mitochondrial superoxide nicotinamide adenine dinucleotide phosphate quinone dehydrogenase (Nqo1) (Moore 2022), which provided a potential therapeutic target to combat obesity and obesity-associated disorders. In bone marrow mesenchymal stem cells from ovariectomized mice, enhanced mitochondrial biogenesis and mitophagy can be mediated via estrogen-related receptor alpha (ERRα), which delays premature cellular senescence, reduces ROS levels, and restores impaired mitochondrial function (Li 2023), suggesting a molecular basis for advancement and development of therapeutic strategies against postmenopausal osteoporosis (PMOP).
In summary, a complex interaction exists between mitochondrial biogenesis and mitophagy to maintain cellular mitochondrial homeostasis. It has been found that regulating mitochondrial biogenesis and mitophagy may become a new direction for the treatment of some diseases, but there are still questions about the mechanism of action of the two, as well as the regulation and feedback loops of their balance. Therefore, further elucidation of how cells maintain optimal mitochondrial populations according to different environmental and developmental conditions, and dissection of the molecular mechanisms of mitochondrial biogenesis or mitophagy for the development of small-molecule drugs targeting both are now urgent questions.
3.2 Mitochondrial dynamics——fission and fusion
Mitochondria are highly dynamic organelles that influence cellular functions by constantly undergoing fusion and fission. Changes in mitochondrial morphology are synergistically regulated by mitochondrial fusion genes (optic atrophy gene 1 (OPA1), MFN1, and MFN2) and mitochondrial fission genes (DRP1) and are involved in a variety of biological processes, including embryonic development, metabolism, apoptosis, and autophagy (Zacharioudakis and Gavathiotis 2023). Mitochondrial fusion replenishes mt DNA loss and equalizes energy utilization, whereas mitochondrial fission removes aged or damaged mitochondria and distributes the normal mitochondrial population (Luan et al. 2021). Therefore, homeostasis of mitochondrial fission and fusion is crucial for mitochondrial quality control and functional maintenance. Excessive mitochondrial fission and fusion can lead to mitochondrial dysfunction and can trigger mitochondria-associated diseases.
Cardiomyocytes exhibit reduced mitochondrial fusion due to excessive mitochondrial division during oxygen-glucose deprivation/reoxygenation (OGD/R) (Guo 2022). The dysregulation of mitochondrial dynamics exacerbates mitochondrial dysfunction and may lead to cardiomyocyte apoptosis, which is a significant risk factor for ischemic heart disease. Recent studies revealed the existence of bidirectional regulatory mechanisms. C-Phycocyanin (C-Pc), an active ingredient extracted from blue-green algae, inhibits the OGD/R-induced overexpression of DRP1, thereby enhancing the expression of MFN1/2 and OPA1 and effectively protecting cardiomyocytes from ischemic injury (Gao 2019). This suggests that mitochondrial kinetic homeostasis has great potential for regulating mitochondrial remodeling and treating mitochondrial disorder-related diseases. In addition, an increase in the ratio of Drp1 and MFN2 protein was found in lung cancer cell lines and samples from lung cancer patients (Fu 2017). Meanwhile, overexpression of MFN2, knockdown of Drp1, or use of Drp1 inhibitors inhibited the proliferation and increased apoptosis of lung cancer cells in vivo and in vitro (Fu 2017). This suggests an imbalance of mitochondrial dynamics in lung cancer. Moreover, excessive mitochondrial fusion and the absence of mitochondrial division have been observed in various cellular senescence models. A recent study showed that cycles of mitochondrial fission and fusion are synchronized with cycles of fatigue and physical recovery induced by a single training session. However, aging reduces the extent to which these parameters change during the exercise/recovery cycle, leading to a decline in fitness, which explains why most anti-aging interventions impair health during aging (Campos 2023). Altered mitochondrial dynamics may not simply be a passive consequence of aging but rather an adaptive mechanism designed to mitigate cellular damage associated with aging, which could serve as a potential target for extending lifespan and promoting healthy aging.
In addition, mitochondria can adapt to different environmental conditions by adjusting their fission/fusion bias. Notably, this bias does not indicate an imbalance but rather maintains a dynamic balance through the regular adaptation of mitochondria to specific conditions. In human white adipocytes (converted to the beige phenotype), DRP1 promotes mitochondrial fission and enhances uncoupled respiration. However, BAT-specific knockout of OPA1 in mice results in mitochondrial dysfunction and loss of BAT thermogenesis (Pisani 2018). This suggests that, despite the tendency of mitochondria to split during thermogenic activity, mitochondrial fusion remains critical for thermogenic function. Mitochondrial carrier 2 (MTCH2)-mediated regulation of mitochondrial elongation is an early driving force in the transition from naïve to primed pluripotency in mouse embryonic stem cells (ESCs) (Bahat 2018). In addition, the transformation of NSCs into neurons is closely linked to mitochondrial dynamics. After mitosis, increased mitochondrial fission promotes neuronal differentiation, whereas the induction of mitochondrial fusion redirects subcellularity toward self-renewal, highlighting the intricate relationship between cellular fate and mitochondrial dynamics (Iwata et al. 2020).
Mitochondrial dynamics play a crucial role in maintaining mitochondrial homeostasis, and any disruption in their balance or impairment of their plasticity may lead to adverse consequences. On the one hand, given the dynamic nature of the fission and fusion processes, enhancing the plasticity of mitochondrial dynamics rather than inhibiting the crosstalk between mitochondrial fusion and fission is a safe and effective strategy for future interventions. However, studies on the mechanisms of bidirectional regulation between division and fusion, and the mechanisms by which they maintain dynamic homeostasis, are limited. On the other hand, some key protein structures have not yet been resolved, and in addition to traditional means of structure resolution, biophysical techniques such as cryo-electron tomography (cryo-ET) and Förster resonance energy transfer (FRET) will also play an important role in mitochondrial fusion studies. In addition, how mitochondrial fusion and splitter protein-mediated fusion of the inner and outer membranes occur in a coordinated manner has also been a focus of attention in the field. The realization of in vitro co-reorganization of the inner and outer membrane fusion machinery, that is, the in vitro reorganization of bilayer membranes, is key to mitochondrial fusion studies. Finally, the study of mitochondrial dynamics should not only focus on the effect of drugs on one of the components but also integrate the concept of "balance" to avoid overly tilting toward one component in practical applications.
3.3 Mitochondrial protein homeostasis
Mitochondria rely on a rigorous system of protein quantity control to accurately perform a range of biological functions. Maintaining the functional integrity of the mitochondrial proteome is also referred to as dynamic protein homeostasis, a specific protein quantity control process that avoids the accumulation of damaged or excess polypeptides. The dynamic equilibrium of mitochondrial proteins is based on endogenous enzyme components, including interrelated chaperone proteins and protein hydrolases, which tightly regulate all stages of import, processing, folding, and assembly of nuclear-encoded mitochondrial proteins, accounting for approximately 99% of total mitochondrial proteins (Kummer and Ban 2021; Song et al. 2021). However, under stressful conditions, many misfolded and denatured peptides may exceed the capacity for cellular regenerative repair. The stress induced by misfolded proteins alters mitochondrial protein translation and proteasome activation. It also initiates the mitochondrial unfolded protein response (UPRmt), which upregulates the expression levels of molecular chaperones, proteases, and other proteases to help proteins return to their normal conformation and reestablish mitochondrial protein homeostasis (Sutandy et al. 2023).
The mitochondrial autophagy receptor protein FUNDC1, localized in the mitochondrial outer membrane, is able to interact with the molecular chaperone protein heat shock cognate70(HSC70), which is localized in the cytosol (Liu 2012). Damaged or misfolded proteins in the cytoplasm can be recruited to the mitochondria through this interaction and later enter the mitochondrial matrix through the translocase of the outer membrane-translocase of the inner membrane complex and are degraded by the matrix-localized mitochondrial protease lon peptidase 1 (LONP1) to help cells maintain protein homeostasis (Li et al. 2019). However, when proteasome activity is inhibited in cells, unfolded proteins accumulate excessively in the mitochondria, compromising mitochondrial integrity, which activates AMPK and leads to the onset of cellular senescence (Li 2019). Neutrophils are particularly sensitive to disturbances in mitochondrial protein homeostasis. The functional caseinolytic peptidase B protein homolog (CLPB)/ HS1-associated protein X-1 (HAX1)/ protein kinase D2 (PRKD2)/heat shock protein 27 (HSP27) axis plays a crucial role in mitochondrial protein homeostasis during human neutrophil differentiation and is a key molecular and metabolic mechanism controlling neutrophil differentiation and function, highlighting a new direction in the development and function of the intrinsic immune system (Fan 2022). It was also found that thermal stimulation enhanced LONP1-mediated succinate dehydrogenase complex iron sulfur subunit B (SDHB) degradation-dependent protein degradation, ensuring adequate intracellular succinate levels and promoting the browning of white adipocytes. Reactivation of the LONP1-succinate pathway in senescent mouse adipocytes restored their conversion to beige adipocytes and improved their adaptive thermogenesis (Fu 2023). In addition, the caseinolytic protease proteolytic subunit (ClpP) facilitates the hydrolysis of misfolded proteins and potentially regulates the UPRmt. The current study identified a novel small-molecule ClpP activator named ZG111 that effectively stimulates the proteolytic function of the ClpP protein, thereby intervening in the UPRmt, and provides a novel approach for combating pancreatic cancer by modulating mitochondrial protein homeostasis (Wang 2020). Moreover, Mitochondrial Misfolded Protein Stress (MMS) transmits signals to the cell nucleus through mtROS and mitochondrial protein precursors, thereby activating the UPRmt71. This elucidates a highly regulated surveillance mechanism in the cytoplasm that integrates distinct mitochondrial stress signals to initiate UPRmt, highlighting the interplay between mitochondrial and cytoplasmic protein homeostasis.
In summary, the maintenance of cellular mitochondrial protein homeostasis is expected to play a pivotal role in regulating the aging and regenerative capacity of an organism, and in combating diseases. In recent years, advances in genomic and proteomic technologies have provided new insights into the various mechanisms that maintain mitochondrial protein homeostasis. Mitochondrial protein homeostasis is governed not only by mitochondrial protein quality control but also by cytoplasmic protein quality control; however, how these two systems are coordinated remains unclear. Furthermore, there is a need to distinguish damaged and functional proteins, particularly in the absence of covalent labeling systems. More information on the range of endogenous substrates is required to establish substrate selection mechanisms and identify protein species that are prone to misfolding.
4 Mitochondria-organelle interaction network
With the emergence of cutting-edge technologies, such as electron cryotomography and real-time super-resolution microscopy, the intricate interactions between mitochondria and many organelles, such as the endoplasmic reticulum (ER), endosomes, lysosomes, and peroxisomes, are gradually being discovered. Mitochondria receive direct support from neighboring organelles that not only provide substrates for reactions but also convey information about the overall cellular state. Therefore, in addition to its function as a "power plant" supplying energy, mitochondria can also act as a cellular signaling station, regulating cell differentiation, apoptosis, and cell cycle at the cellular level through the processes of sensing, integration, and signal modulation (Fig. 4).
Mitochondria-organelle interaction network. Mitochondria receive direct support from neighboring organelles, such as endoplasmic reticulum, endosomes, lysosomes, and peroxisomes, that not only provide substrates for reactions but also convey information about the overall cellular state. Mitochondrial and cellular homeostasis are regulated by the mitochondria-organelle interaction network. Created with Biorender
The most studied mitochondrial-organelle interactions occur with the ER. There is a direct interaction between mitochondria and the ER, and the membrane structures that mediate mitochondria-ER interactions are called mitochondria-associated membranes (MAMs). Their significance in maintaining mitochondrial homeostasis and determining cell fate has been increasingly recognized (Jiang et al. 2023). MAMs have been found to exist in large numbers in different tissues and cell types and play a considerable role in regulating the transport of calcium ions (Ca2+) between the mitochondria and ER, the maintenance of mitochondrial morphology, the production of cellular bioenergy, lipid metabolism and transport, and ER stress (Ji 2022). In the tumor-infiltrating cluster of differentiation 8 (CD8)+ T cells, mitofusin 2 (MFN2) enhances mitochondrial-endoplasmic reticulum contact by interacting with the ER-embedded sarcoplasmic/ER Ca2+ ATP hydrolase 2 (SERCA2), which promotes efficient mitochondrial Ca2+ influx required by the mitochondria. Enhancing the mitochondria-ER contact by increasing MFN2 level in CD8+ T cells improves the efficacy of cancer immunotherapy, reveals a buffering mechanism that regulates metabolic adaptations in tumor-infiltrating CD8+ T cells, and highlights the therapeutic potential of optimizing T cell function by increasing MFN2 expression (Yang 2023). The crosstalk between zinc ion (Zn2+) in the ER and mitochondria maintains mitochondrial homeostasis. The ER harbors a pool of Zn2+ for mitochondrial uptake. A novel autosomal recessive cerebro-renal syndrome was identified in consanguineous Bedouin kindred and its pathogenesis is related to SLC30A9 (an exon with properties that affect intracellular zinc homeostasis) mutation. The current study demonstrated that reducing the solute carrier family 30 member (SLC30A)5-mediated import of Zn2+ into the ER can ameliorate structural and functional defects in mitochondria by inhibiting the accumulation of mitochondrial Zn2+ mediated by SLC30A9 mutants (Perez 2017). Moreover, alterations in Ca2+ flux mediated by MAM proteins due to MAM formation impede tumor and stromal cell mitochondrial metabolism within the tumor microenvironment. Thioredoxin-related transmembrane protein 1 (TMX1) interacts with SERCA2b to enhance ATP production in mitochondria through the regulation of ER-mitochondrial contacts and Ca2+ flux at MAMs, providing a foundation for combating tumors via mitochondria-ER crosstalk (Raturi 2016). Additionally, the ER's involvement in mitochondria extends to organelle division. Mitochondrial division takes place at locations where ER tubules make contact with mitochondria, orchestrating constriction before the recruitment of Drp1 (Friedman 2011), demonstrating that ER tubules actively contribute to determining the positions of mitochondrial division sites. Finally, ABHD16A, an ER phospholipid hydrolase, functions by altering phospholipid composition at ER-mitochondria membrane contacting sites (MSCs), regulating the recruitment of both fission and fusion machinery to mitochondria, indicating the role of ER in both mitochondrial fission and fusion (Nguyen 2022). Moreover, dysfunction of MAM function is associated with non-alcoholic fatty liver disease. Mitofusion2 (Mfn2), in addition to being a key protein in regulating mitochondrial dynamics, is one of the proteins that physically link the structure of MAMs. The specific deletion of Mfn2 in mouse liver will lead to liver inflammation and lipid metabolism disorders. Additionally, Mfn2 deletion leads to increased apoptosis, accelerated cell proliferation of mice liver cells, and even fibrosis and cancer of the whole liver (Barbier-Torres 2020). Collectively, these studies underscore the intricate and indispensable nature of the ER-mitochondria relationship, demonstrating its broad impact on cellular homeostasis and functionality.
In endocytosis, extracellular macromolecules form endosomes that fuse with lysosomes for degradation. Mitochondria interplay with the endocytic system, and specific extracellular proteins like anti-angiogenic Angiostatin (Lee 2009)and Isthmin (Chen 2014), Helicobacter pylori VacA toxin (Calore 2010), and cell surface receptors such as Epidermal Growth Factor Receptor (EGFR) (Demory 2009; Yao 2010; Cao et al. 2011)and Glucose-Regulated Protein 78 kDa (GRP78) (Moser 1999; Chi and Pizzo 2006)can be transported to mitochondria via endocytosis. Moreover, conventional mitochondrial proteins like ATP Synthase (Moser 1999; Chi and Pizzo 2006)and Voltage-Dependent Anion-Selective Channel (VDAC) can translocate to the cell surface, acting as receptors (De Pinto et al. 2010). These findings suggest a protein transport mechanism linking extracellular elements with mitochondria through the endocytic route. Indeed, Mitochondria directly interact with endosomes, likely through membrane fusion, engaging in lipid, protein, and ion exchange (Calore 2010; Charman et al. 2010; Das et al. 2016; Chen 2018). Investigations into the machinery at the mitochondria-endosome membrane contact site are ongoing, aiming to elucidate the mechanism of mitochondria and endosome interaction and the role of the endocytic system in sustaining mitochondrial homeostasis.
Aligned with the link to endosomes, there are also well-coordinated interactions between lysosomes and mitochondria that ensure the stability of the intracellular environment and help maintain normal physiological cellular functions (Deus et al. 2020). Mitochondria and lysosomes often establish transient and continuous contacts, also known as mitochondria-lysosome contact under physiological and pathological conditions, allowing the exchange of lipids, proteins, ions, and other molecules between them and triggering mitochondrial division via lysosomes (Khaddaj and Kukulski 2023). These interactions are important for the regulation of mitochondrial function, autophagy, and cell signaling pathways. Aberrant organelle interactions are closely associated with cell death and various diseases. RAB7-guanosine triphosphate (GTP) on lysosomes promotes contact formation and stabilization, and the two are then dissociated by fission 1 (FIS1), which recruits TBC1 domain family member 15 (TBC1D15) to the mitochondria to exert Rab7 GTP hydrolase activity. Hydrolysis of Rab7-GTP is regulated by TBC1D15 in the mitochondria, which provides a mechanism by which mitochondria regulate lysosomal dynamics by turning off RAB7 activity. Overexpression of TBC1D15 alleviated the accumulation of damaged mitochondria in cells after myocardial infarction, which was associated with impaired mitochondrial clearance due to defective lysosomal enlargement and the subsequent disruption of autophagic flow (Wong et al. 2018). Furthermore, lysosomes play a crucial role in supporting mitochondrial function by providing essential amino acids (Nowosad 2022). However, in specific forms of Parkinson's disease, the interaction between lysosomes and mitochondria is disrupted, impairing their normal function, leading to mitochondrial dysfunction and ultimately resulting in the degeneration of vulnerable neurons associated with Parkinson's disease (Peng 2023). Restoring the contact between the mitochondria and lysosomes has emerged as a potential therapeutic strategy for treating this condition. Undergoing morphological changes into giant structures facilitates mitochondrial self-digestion through enhanced interaction between lysosomes and mitochondria as well as megamitochondria engulfing lysosome (Hao 2023). This sheds light on a novel mode and mechanism of communication between mitochondria and endolysosomes while uncovering an alternative pathway for maintaining cellular homeostasis.
Peroxisomes play crucial roles in scavenging ROS and maintaining cellular homeostasis. Functionally, peroxisomes are interconnected with mitochondria, forming the so-called "peroxisome-mitochondria connection" (Fransen 2017). Peroxisomes and mitochondria cooperate to perform basic metabolic functions in the cell, including fatty acid oxidation and maintenance of the redox homeostasis (Jiang and Okazaki 2022). In particular, peroxisomal biogenesis factor (Pex)3 and Pex5 play key roles in peroxisome biogenesis, and inhibition of gene expression induces mitochondrial fragmentation mediated by DRP1 in mouse embryonic fibroblasts (Tanaka 2019). In addition, peroxisomes significantly enhanced etoposide-triggered caspase activation and apoptosis, thereby enhancing the cellular susceptibility to death signals, suggesting a regulatory role for peroxisomes in mitochondrial dynamics and mitochondria-dependent apoptosis (Tanaka 2019). The regulatory role of peroxisomes in mitochondrial function during myogenesis has also been confirmed. The regulatory role of peroxisomes in mitochondrial function was also confirmed during myogenesis. Knockdown of Pex3 not only hinders the density and function of peroxisomes but also inhibits the expression and activity of mitochondrial genes, suggesting a close interaction between peroxisome biogenesis and mitochondrial function (Wu 2023).
The signaling role of mitochondria is increasingly being recognized; thus, it is important to study the interrelationships between mitochondria and other organelles in depth. This will not only help to reveal the specific mechanisms of disease development but will also further accelerate the emergence of new approaches to treating diseases by explicitly regulating mitochondrial homeostasis. However, the complete network of interactions between mitochondria and other organelles remains unclear. Therefore, elucidating a comprehensive map of these interactions should be a focus of future biological research, not only for mitochondrial homeostasis but also for cellular and organismal stability.
5 Mitochondria-nucleus communication
Mitochondria possess their own genome, which encodes 13 proteins, whereas the majority (approximately 1,200) of proteins are encoded by nuclear genes (Soledad et al. 2019). Consequently, close coordination and communication between the nuclear and mitochondrial genomes are essential to effectively regulate their activities, ensuring adaptive mitochondrial function in response to dynamic cellular environments and maintaining mitochondrial homeostasis (Quiros et al. 2016). Mitochondrial dysfunction leads to the accumulation of unfolded or misfolded proteins, which can be recognized by molecular chaperones and transported to the nucleus to induce the activation of the corresponding transcription factors and repair mitochondrial damage (Sutandy et al. 2023). Moreover, mitochondrial metabolites can participate directly or indirectly in epigenetic modifications, thereby regulating mitochondrial protein expression. Recent research has revealed that early in mammalian development, cell lineage-specific mitochondrial gene expression is manifested. Distinct mtDNA mutations can disrupt intramitochondrial protein synthesis, thereby triggering specific compensatory responses within the affected cell lineages (Burr 2023). This evidence supports the establishment of mitochondria-nucleus crosstalk long before organ maturation (Fig. 5).
Mitochondria-nucleus communication. Mitochondrial dysfunction can trigger the activation of corresponding transcription factors through ISR-UPR, facilitating the repair of mitochondrial damage. Moreover, mitochondrial metabolites can directly or indirectly participate in epigenetic modifications, thereby regulating the expression of mitochondrial proteins. The close coordination and communication between the nucleus and mitochondria play a crucial role in effectively regulating mitochondrial function and maintaining its normal operation. ISR, integrated stress response; UPRmt, mitochondrial unfolded protein response; Ac, Acetoxy; Ub, ubiquitin; Me, methyl; TF, transcription factors; ROS, reactive oxygen species. Created with Biorender
In mammals, the integrated stress response (ISR) and UPRmtare primary examples of nuclear-mitochondrial crosstalk mechanisms that promote mitochondrial homeostasis. Kinases such as general control nonderepressible 2 (GCN2), protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK), heme-regulated inhibitor (HRI), and PKR could phosphorylate serine residues on the α subunit of eukaryotic initiation factor 2 (eIF-2) by activation of mitochondrial dysfunction (Hamada 2020). This activation subsequently triggers ISR and counteracts the inhibitory effect of upstream open reading frames on downstream molecules C/EBP homologous protein (CHOP), activating transcription factor (ATF)4, and ATF5 (Melber and Haynes 2018). Consequently, the UPRmtis activated, leading to a reduction in total protein translation and an increase in the synthesis of proteins involved in environmental adaptation, to achieve functional mitochondrial homeostasis (Read 2021). In addition, mitochondrial stress activates OMA1-mediated cleavage of the death-associated protein 3 (DAP3) binding cell death enhancer 1 (DELE1) protein, in turn, activates HRI, leading to increased expression of ATF4. Mitochondria appear to send distress signals to the nucleus through the OMA1-DELE1-HRI pathway, which activates a series of mechanisms to counteract stress and improve mitochondrial function (Guo 2020).
Moreover, a variety of metabolites of the TCA cycle have been demonstrated to regulate epigenetic modifications. For instance, acetyl-CoA serves as a substrate for protein acetylation modification, S-adenosylmethionine (SAM) influences histone and DNA methylation, and α-ketoglutarate (α-KG) acts as a crucial regulator of histone and DNA demethylation(Tran et al. 2019; Ouyang et al. 2020; Shvedunova and Akhtar 2022). Pyruvate dehydrogenase E1 subunit alpha 1 (Pdha1) promotes the transformation of ESCs into two-cell-like embryonic cells through a mechanism whereby the nuclear import of Pdha1 facilitates the direct synthesis of acetyl-CoA in the nucleus, providing a reaction substrate for histone acetylation modification and promoting histone H3 acetylation(Li 2022). Nuclear localization of Pdha1 enhances binding between P300 and the pluripotency core regulators SRY-box transcription factor 2 (Sox2)/ Krüppel-like factor 4 (Klf4)/ octamer-binding transcription factor 4 (Oct4) at transcriptional start sites and enhancer regions of pluripotency genes by elevating the levels of H3K9ac and H3K27ac modifications, thus facilitating the establishment of the pluripotency gene network(Li 2022).
In addition to the metabolites of the TCA cycle, ROS also promotes mitochondria-nucleus crosstalk. In cancer chemotherapy, certain broad-spectrum anticancer drugs exploit the cytotoxicity of ROS to induce cancer cell apoptosis by upregulating intracellular ROS levels(Perillo 2020). The nuclear sensor of ROS, checkpoint kinase 1 (CHK1)-C408, which refers to the oxidative modification of the CHK1 protein at position C408, modulates the expression of single-stranded DNA binding protein 1 (SSBP1), an active mtDNA-binding protein in the mitochondria, and regulates nuclear H2O2 levels by controlling mitochondrial translation(Zhang 2023). Therefore, the combination of CHK1 inhibitors and platinum-based drugs is a promising therapeutic approach for overcoming platinum-based drug resistance.
Accumulating evidence supports the important role of mitochondria-nucleus communication in the regulation of mitochondrial homeostasis. However, several questions remain unanswered. What are the specific epigenetic factors involved in mitochondrial-nucleus communication? How do these factors cooperate with transcription factors in response to various external and internal stimuli? What are the downstream regulatory pathways of CHOP, ATF4, and ATF5? How do they regulate the UPRmt? It is unclear whether and how the key proteins involved in mitochondrion-nucleus communication cooperate to regulate mitochondrial homeostasis. Further in-depth studies are required to provide reliable theoretical support for new targeted therapies for mitochondrial disorder-related diseases.
6 Conclusion and prospects
Mitochondria are important cellular organelles, with the preservation of mitochondrial homeostasis proving indispensable for regular cell function and overall survival. Delving into the regulatory mechanisms governing mitochondrial homeostasis not only unveils the inherent biological properties and functions of mitochondria but also furnishes a theoretical foundation and guidance for manipulating cell growth and cycle regulation in the realm of cell biology research. Moreover, this exploration deepens our comprehension of the pathogenesis underlying mitochondria-related diseases, thereby contributing to advancements in biomedicine.
However, numerous aspects remain elusive, prompting the need for future research in the following directions:
-
1.
The intricate and sophisticated network of mitochondrial molecular mechanisms necessitates the integration of sequencing, omics, and artificial intelligence to construct a comprehensive mitochondrial molecular regulatory network, thereby enabling a more intuitive and comprehensive understanding of the regulatory mechanisms underlying mitochondrial homeostasis.
-
2.
The mitochondria undergo constant changes. Therefore, the focus of mitochondrial targeting should be on maintaining homeostasis rather than solely regulating independent mitochondrial processes. Similarly, future research on mitochondrial regulation should prioritize the investigation of molecular mechanisms responsible for sustaining mitochondrial homeostasis.
-
3.
Mitochondria possess autonomous quality control systems that enable self-repair and rejuvenation within their physiological spectra. In future studies, it will be imperative to further explore strategies for maximizing the regulatory potential of mitochondria in governing cellular and systemic processes, thereby facilitating safer and more efficacious approaches toward the prevention and treatment of associated disorders.
-
4.
Currently, the availability of drugs that specifically target mitochondria remains limited, and their safety profile remains controversial. Therefore, there is an urgent need to develop safe, precise, and effective mitochondria-targeting drugs.
Homeostasis serves as an indicator of organismal health, with normal homeostatic regulation forming the basis for maintaining overall health. In recent years, an increasing number of adverse conditions and diseases, including Parkinson’s disease, cancer, inflammation, and aging, have been strongly associated with dysregulated mitochondrial homeostasis. Therefore, the regulatory mechanism of cellular mitochondrial homeostasis is a worthy focus in the research of homeostatic medicine.
Availability of data and materials
NA.
References
Aranda-Rivera AK, Cruz-Gregorio A, Aparicio-Trejo OE, Pedraza-Chaverri J. Mitochondrial Redox Signaling and Oxidative Stress in Kidney Diseases. Biomolecules. 2021;11:1144.
Anderson AJ, Jackson TD, Stroud DA, Stojanovski D. Mitochondria-hubs for regulating cellular biochemistry: emerging concepts and networks. Open Biol. 2019;9:190126.
Bahat A, et al. MTCH2-mediated mitochondrial fusion drives exit from naive pluripotency in embryonic stem cells. Nat Commun. 2018;9:5132.
Barbier-Torres L, et al. Silencing hepatic MCJ attenuates non-alcoholic fatty liver disease (NAFLD) by increasing mitochondrial fatty acid oxidation. Nat Commun. 2020;11:3360.
Burr SP, et al. Cell lineage-specific mitochondrial resilience during mammalian organogenesis. Cell. 2023;186:1212-1229 e1221.
Calore F, et al. Endosome-mitochondria juxtaposition during apoptosis induced by H. pylori VacA. Cell Death Differ. 2010;17:1707–1716.
Campos JC, et al. Exercise preserves physical fitness during aging through AMPK and mitochondrial dynamics. Proc Natl Acad Sci U S A. 2023;120: e2204750120.
Cao X, Zhu H, Ali-Osman F, Lo HW. EGFR and EGFRvIII undergo stress- and EGFR kinase inhibitor-induced mitochondrial translocalization: a potential mechanism of EGFR-driven antagonism of apoptosis. Mol Cancer. 2011;10:26.
Carter JL, et al. Targeting mitochondrial respiration for the treatment of acute myeloid leukemia. Biochem Pharmacol. 2020;182: 114253.
Charman M, Kennedy BE, Osborne N, Karten B. MLN64 mediates egress of cholesterol from endosomes to mitochondria in the absence of functional Niemann-Pick Type C1 protein. J Lipid Res. 2010;51:1023–1034.
Chen M, et al. Extracellular anti-angiogenic proteins augment an endosomal protein trafficking pathway to reach mitochondria and execute apoptosis in HUVECs. Cell Death Differ. 2018;25:1905–1920.
Chen M, et al. Isthmin targets cell-surface GRP78 and triggers apoptosis via induction of mitochondrial dysfunction. Cell Death Differ. 2014;21:797–810.
Chen Y, Liang Y, Luo X, Hu Q. Oxidative resistance of leukemic stem cells and oxidative damage to hematopoietic stem cells under pro-oxidative therapy. Cell Death Dis. 2020;11:291.
Chi SL, Pizzo SV. Cell surface F1Fo ATP synthase: a new paradigm? Ann Med. 2006;38:429–438.
Cohen P, Kajimura S. The cellular and functional complexity of thermogenic fat. Nat Rev Mol Cell Biol. 2021;22:393–409.
Cui M, et al. Nrf1 promotes heart regeneration and repair by regulating proteostasis and redox balance. Nat Commun. 2021;12:5270.
Das A, Nag S, Mason AB, Barroso MM. Endosome-mitochondria interactions are modulated by iron release from transferrin. J Cell Biol. 2016;214:831–845.
Demory ML, et al. Epidermal growth factor receptor translocation to the mitochondria: regulation and effect. J Biol Chem. 2009;284:36592–36604.
De Pinto V, Messina A, Lane DJ, Lawen A. Voltage-dependent anion-selective channel (VDAC) in the plasma membrane. FEBS Lett. 2010;584:1793–1799.
Deus CM, Yambire KF, Oliveira PJ, Raimundo N. Mitochondria-Lysosome Crosstalk: From Physiology to Neurodegeneration. Trends Mol Med. 2020;26:71–88.
Eiyama A, Okamoto K. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr Opin Cell Biol. 2015;33:95–101.
Esteras N, Abramov AY. Nrf2 as a regulator of mitochondrial function: Energy metabolism and beyond. Free Radic Biol Med. 2022;189:136–153.
Fan Y, et al. HAX1-dependent control of mitochondrial proteostasis governs neutrophil granulocyte differentiation. J Clin Invest. 2022;132:e153153.
Fransen M, Lismont C, Walton P. The Peroxisome-Mitochondria Connection: How and Why? Int J Mol Sci. 2017;18:1126.
Friedman JR, et al. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362.
Fu T, et al. Proteolytic rewiring of mitochondria by LONP1 directs cell identity switching of adipocytes. Nat Cell Biol. 2023;25:848–864.
Fiocca R, et al. Widespread expression of intestinal markers in gastric carcinoma: a light and electron microscopic study using BD-5 monoclonal antibody. J Clin Pathol. 1988;41:178–187.
Fu L, et al. SIRT4 inhibits malignancy progression of NSCLCs, through mitochondrial dynamics mediated by the ERK-Drp1 pathway. Oncogene. 2017;36:2724–2736.
Gajwani P, Rehman J. MitoSwap - Mitophagy partnered with compensatory mitochondrial biogenesis during stem cell differentiation. Autophagy Rep. 2022;1:210–213.
Gao J, et al. C-Phycocyanin Ameliorates Mitochondrial Fission and Fusion Dynamics in Ischemic Cardiomyocyte Damage. Front Pharmacol. 2019;10:733.
Gensbittel V, et al. Mechanical Adaptability of Tumor Cells in Metastasis. Dev Cell. 2021;56:164–179.
Grassian AR, Metallo CM, Coloff JL, Stephanopoulos G, Brugge JS. Erk regulation of pyruvate dehydrogenase flux through PDK4 modulates cell proliferation. Genes Dev. 2011;25:1716–1733.
Guo H, et al. ZFP36 protects against oxygen-glucose deprivation/reoxygenation-induced mitochondrial fragmentation and neuronal apoptosis through inhibiting NOX4-DRP1 pathway. Brain Res Bull. 2022;179:57–67.
Guo X, et al. Mitochondrial stress is relayed to the cytosol by an OMA1-DELE1-HRI pathway. Nature. 2020;579:427–432.
Hamada Y, et al. Nanosecond pulsed electric fields induce the integrated stress response via reactive oxygen species-mediated heme-regulated inhibitor (HRI) activation. PLoS ONE. 2020;15: e0229948.
Handy DE, Loscalzo J. Redox regulation of mitochondrial function. Antioxid Redox Signal. 2012;16:1323–1367.
Hao T, et al. Hypoxia-reprogramed megamitochondrion contacts and engulfs lysosome to mediate mitochondrial self-digestion. Nat Commun. 2023;14:4105.
Harrington JS, Ryter SW, Plataki M, Price DR, Choi AMK. Mitochondria in health, disease, and aging. Physiol Rev. 2023;103:2349–2422.
Hu S, et al. Nrf1 is an indispensable redox-determining factor for mitochondrial homeostasis by integrating multi-hierarchical regulatory networks. Redox Biol. 2022;57: 102470.
Huang Z, Chen Y, Zhang Y. Mitochondrial reactive oxygen species cause major oxidative mitochondrial DNA damages and repair pathways. J Biosci. 2020;45:84.
Iwata R, Casimir P, Vanderhaeghen P. Mitochondrial dynamics in postmitotic cells regulate neurogenesis. Science. 2020;369:858–862.
Jardim-Messeder D, et al. Fumarate reductase superfamily: A diverse group of enzymes whose evolution is correlated to the establishment of different metabolic pathways. Mitochondrion. 2017;34:56–66.
Ji C, et al. Mitochondria-Associated Endoplasmic Reticulum Membranes: Inextricably Linked with Autophagy Process. Oxid Med Cell Longev. 2022;2022:7086807.
Jia L, et al. Rheb-regulated mitochondrial pyruvate metabolism of Schwann cells linked to axon stability. Dev Cell. 2021;56:2980-2994 e2986.
Jiang C, Okazaki T. Control of mitochondrial dynamics and apoptotic pathways by peroxisomes. Front Cell Dev Biol. 2022;10: 938177.
Jiang RQ, Li QQ, Sheng R. Mitochondria associated ER membranes and cerebral ischemia: Molecular mechanisms and therapeutic strategies. Pharmacol Res. 2023;191: 106761.
Jiang X, et al. Targeting PI4KA sensitizes refractory leukemia to chemotherapy by modulating the ERK/AMPK/OXPHOS axis. Theranostics. 2022;12:6972–6988.
Jin L, et al. Mitophagy induced by UMI-77 preserves mitochondrial fitness in renal tubular epithelial cells and alleviates renal fibrosis. FASEB J. 2022;36: e22342.
Jing M, et al. Celastrol inhibits rheumatoid arthritis through the ROS-NF-kappaB-NLRP3 inflammasome axis. Int Immunopharmacol. 2021;98: 107879.
Kamarajugadda S, et al. Glucose oxidation modulates anoikis and tumor metastasis. Mol Cell Biol. 2012;32:1893–1907.
Khaddaj R, Kukulski W. Piecing together the structural organisation of lipid exchange at membrane contact sites. Curr Opin Cell Biol. 2023;83: 102212.
Krantz S, et al. Mitophagy mediates metabolic reprogramming of induced pluripotent stem cells undergoing endothelial differentiation. J Biol Chem. 2021;297: 101410.
Kummer E, Ban N. Mechanisms and regulation of protein synthesis in mitochondria. Nat Rev Mol Cell Biol. 2021;22:307–325.
Lee TY, et al. Angiostatin regulates the expression of antiangiogenic and proapoptotic pathways via targeted inhibition of mitochondrial proteins. Blood. 2009;114:1987–1998.
Li M, et al. Genistein mitigates senescence of bone marrow mesenchymal stem cells via ERRalpha-mediated mitochondrial biogenesis and mitophagy in ovariectomized rats. Redox Biol. 2023;61: 102649.
Li S, et al. Drp1-regulated PARK2-dependent mitophagy protects against renal fibrosis in unilateral ureteral obstruction. Free Radic Biol Med. 2020;152:632–649.
Li T, et al. PKM2 coordinates glycolysis with mitochondrial fusion and oxidative phosphorylation. Protein Cell. 2019a;10:583–594.
Li W, et al. Nuclear localization of mitochondrial TCA cycle enzymes modulates pluripotency via histone acetylation. Nat Commun. 2022;13:7414.
Li Y, Liu L, Zhu Y, Chen Q. Mitochondria organize the cellular proteostatic response and promote cellular senescence. Cell Stress. 2019b;3:110–114.
Li Y, et al. A mitochondrial FUNDC1/HSC70 interaction organizes the proteostatic stress response at the risk of cell morbidity. EMBO J. 2019;38:e98786.
Li Y, et al. The Role of Mitophagy in Viral Infection. Cells. 2022;11:711.
Li Y, et al. Local hyperthermia therapy induces browning of white fat and treats obesity. Cell. 2022;185:949-966 e919.
Liu L, et al. Mitophagy receptor FUNDC1 is regulated by PGC-1alpha/NRF1 to fine tune mitochondrial homeostasis. EMBO Rep. 2021;22: e50629.
Liu L, et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012;14:177–185.
Liu Y, Birsoy K. Metabolic sensing and control in mitochondria. Mol Cell. 2023;83:877–889.
Luan Y, Ren KD, Luan Y, Chen X, Yang Y. Mitochondrial Dynamics: Pathogenesis and Therapeutic Targets of Vascular Diseases. Front Cardiovasc Med. 2021;8: 770574.
Lv M, et al. Adipose-derived stem cells regulate metabolic homeostasis and delay aging by promoting mitophagy. FASEB J. 2021;35: e21709.
Manford AG, et al. A Cellular Mechanism to Detect and Alleviate Reductive Stress. Cell. 2020;183:46-61 e21.
Martinez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat Rev Cancer. 2021;21:669–680.
Melber A, Haynes CM. UPR(mt) regulation and output: a stress response mediated by mitochondrial-nuclear communication. Cell Res. 2018;28:281–295.
Meng Q, et al. Rosiglitazone Ameliorates Spinal Cord Injury via Inhibiting Mitophagy and Inflammation of Neural Stem Cells. Oxid Med Cell Longev. 2022;2022:5583512.
Miranda-Vizuete A, Veal EA. Caenorhabditis elegans as a model for understanding ROS function in physiology and disease. Redox Biol. 2017;11:708–714.
Moore TM, et al. Parkin regulates adiposity by coordinating mitophagy with mitochondrial biogenesis in white adipocytes. Nat Commun. 2022;13:6661.
Moser TL, et al. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci U S A. 1999;96:2811–2816.
Nanadikar MS, et al. IDH3gamma functions as a redox switch regulating mitochondrial energy metabolism and contractility in the heart. Nat Commun. 2023;14:2123.
Narasimhan KKS, et al. Reductive stress promotes protein aggregation and impairs neurogenesis. Redox Biol. 2020;37:101739.
Ni HM, Williams JA, Ding WX. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015;4:6–13.
Nguyen TT, Voeltz GK. An ER phospholipid hydrolase drives ER-associated mitochondrial constriction for fission and fusion. Elife. 2022;11:e84279.
Nowosad A, Besson A. Lysosomes at the Crossroads of Cell Metabolism, Cell Cycle, and Stemness. Int J Mol Sci. 2022;23:2290.
Ouyang Y, Wu Q, Li J, Sun S, Sun S. S-adenosylmethionine: A metabolite critical to the regulation of autophagy. Cell Prolif. 2020;53: e12891.
Perillo B, et al. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52:192–203.
Peng W, Schroder LF, Song P, Wong YC, Krainc D. Parkin regulates amino acid homeostasis at mitochondria-lysosome (M/L) contact sites in Parkinson’s disease. Sci Adv. 2023;9:eadh3347.
Perez Y, et al. SLC30A9 mutation affecting intracellular zinc homeostasis causes a novel cerebro-renal syndrome. Brain. 2017;140:928–939.
Picard M, Shirihai OS. Mitochondrial signal transduction. Cell Metab. 2022;34:1620–1653.
Pisani DF, et al. Mitochondrial fission is associated with UCP1 activity in human brite/beige adipocytes. Mol Metab. 2018;7:35–44.
Quiros PM, Mottis A, Auwerx J. Mitonuclear communication in homeostasis and stress. Nat Rev Mol Cell Biol. 2016;17:213–226.
Raturi A, et al. TMX1 determines cancer cell metabolism as a thiol-based modulator of ER-mitochondria Ca2+ flux. J Cell Biol. 2016;214:433–444.
Razzoli M, Emmett MJ, Lazar MA, Bartolomucci A. beta-Adrenergic receptors control brown adipose UCP-1 tone and cold response without affecting its circadian rhythmicity. FASEB J. 2018;32:5640–5646.
Read A, Schroder M. The Unfolded Protein Response: An Overview. Biology (Basel). 2021;10:384.
Roca-Portoles A, Tait SWG. Mitochondrial quality control: from molecule to organelle. Cell Mol Life Sci. 2021;78:3853–3866.
Romani P, et al. Mitochondrial fission links ECM mechanotransduction to metabolic redox homeostasis and metastatic chemotherapy resistance. Nat Cell Biol. 2022;24:168–180.
Ruccolo S, et al. Electrochemical Recycling of Adenosine Triphosphate in Biocatalytic Reaction Cascades. J Am Chem Soc. 2022;144:22582–22588.
Schirrmacher V. Mitochondria at Work: New Insights into Regulation and Dysregulation of Cellular Energy Supply and Metabolism. Biomedicines. 2020;8:526.
Shvedunova M, Akhtar A. Modulation of cellular processes by histone and non-histone protein acetylation. Nat Rev Mol Cell Biol. 2022;23:329–349.
Soledad RB, Charles S, Samarjit D. The secret messages between mitochondria and nucleus in muscle cell biology. Arch Biochem Biophys. 2019;666:52–62.
Song J, Herrmann JM, Becker T. Quality control of the mitochondrial proteome. Nat Rev Mol Cell Biol. 2021;22:54–70.
Spinelli JB, Haigis MC. The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol. 2018;20:745–754.
Sutandy FXR, Gossner I, Tascher G, Munch C. A cytosolic surveillance mechanism activates the mitochondrial UPR. Nature. 2023;618:849–854.
Tanaka H, et al. Peroxisomes control mitochondrial dynamics and the mitochondrion-dependent apoptosis pathway. J Cell Sci. 2019;132:jcs224766.
Tran KA, Dillingham CM, Sridharan R. The role of alpha-ketoglutarate-dependent proteins in pluripotency acquisition and maintenance. J Biol Chem. 2019;294:5408–5419.
Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79:208–217.
Vultaggio-Poma V, Sarti AC, Di Virgilio F. Extracellular ATP: A Feasible Target for Cancer Therapy. Cells. 2020;9:2496.
Wang F, Zhang D, Zhang D, Li P, Gao Y. Mitochondrial Protein Translation: Emerging Roles and Clinical Significance in Disease. Front Cell Dev Biol. 2021;9: 675465.
Wang N, et al. PINK1: The guard of mitochondria. Life Sci. 2020;259: 118247.
Wang S, Qin L. Homeostatic medicine: a strategy for exploring health and disease. Curr Med (Cham). 2022;1:16.
Wang YP, et al. Malic enzyme 2 connects the Krebs cycle intermediate fumarate to mitochondrial biogenesis. Cell Metab. 2021;33:1027-1041 e1028.
Wasner K, et al. Parkin Deficiency Impairs Mitochondrial DNA Dynamics and Propagates Inflammation. Mov Disord. 2022;37:1405–1415.
Wong YC, Kim S, Peng W, Krainc D. Regulation and Function of Mitochondria-Lysosome Membrane Contact Sites in Cellular Homeostasis. Trends Cell Biol. 2019;29:500–513.
Wong YC, Ysselstein D, Krainc D. Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature. 2018;554:382–386.
Wu CC, et al. Reciprocal Regulation of Peroxisome Biogenesis and Myogenic Factors Is Critical for Myogenesis. Int J Mol Sci. 2023;24:12262.
Yang JF, et al. Mitochondria-ER contact mediated by MFN2-SERCA2 interaction supports CD8(+) T cell metabolic fitness and function in tumors. Sci Immunol. 2023;8:eabq2424.
Yao YA, et al. Mitochondrially localized EGFR is independent of its endocytosis and associates with cell viability. Acta Bioch Bioph Sin. 2010;42:763–770.
Yu M, Wang D, Chen X, Zhong D, Luo J. BMSCs-derived Mitochondria Improve Osteoarthritis by Ameliorating Mitochondrial Dysfunction and Promoting Mitochondrial Biogenesis in Chondrocytes. Stem Cell Rev Rep. 2022;18:3092–3111.
Zacharioudakis E, Gavathiotis E. Mitochondrial dynamics proteins as emerging drug targets. Trends Pharmacol Sci. 2023;44:112–127.
Zeng W, et al. Restoration of CPEB4 prevents muscle stem cell senescence during aging. Dev Cell. 2023;58:1383-1398 e1386.
Zhang J, et al. Systematic identification of anticancer drug targets reveals a nucleus-to-mitochondria ROS-sensing pathway. Cell. 2023;186:2361-2379 e2325.
Zhao Q, et al. ZNF281 inhibits mitochondrial biogenesis to facilitate metastasis of hepatocellular carcinoma. Cell Death Discov. 2023;9:396.
Zhou H, Ren J, Toan S, Mui D. Role of mitochondrial quality surveillance in myocardial infarction: From bench to bedside. Ageing Res Rev. 2021;66: 101250.
Zhu J, Thompson CB. Metabolic regulation of cell growth and proliferation. Nat Rev Mol Cell Biol. 2019;20:436–450.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (82030031, L2224038, 92149301); Chinese Academy of Medical Sciences Research Unit (2019-12M-5-031); Beijing Municipal Science & Technology Commission (Z181100001718208); Beijing Municipal Education Commission (119207020201); Beijing Municipality Government Grants (Beijing Laboratory of Oral Health-PXM2021_014226_000041, Beijing Scholar Program-PXM2021_014226_000020); Innovation Research Team Project of Beijing Stomatological Hospital, Capital Medical University (CXTD202201); Young Scientist Program of Beijing Stomatological Hospital, Capital Medical University (YSP202308).
Funding
None.
Author information
Authors and Affiliations
Contributions
Q.L. and J.O. wrote the manuscript, C.M. revised the manuscript, and W.S. guided and revised the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
NA.
Consent for publication
All authors agree to publish.
Competing interests
The author Songlin Wang is a member of the Editorial Board for Current Medicine. The paper was handled by the other journal Editor and has undergone rigorous peer review process. Songlin Wang was not involved in the journal’s review of, or decisions related to, this manuscript. The other authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, X., Jiang, O., Chen, M. et al. Mitochondrial homeostasis: shaping health and disease. Curr Med 3, 5 (2024). https://doi.org/10.1007/s44194-024-00032-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44194-024-00032-x