Abstract
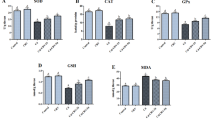
Cadmium (Cd) is a well-known heavy metal and a neurotoxic agent. Minocycline (Mino) is an anti-microbial agent with a lipophilic structure that crosses the blood–brain barrier and enters the cerebral tissue. In recent studies, Mino has been introduced as an antioxidant and anti-apoptotic chemical compound, and therefore, it was examined as a protective candidate against Cd-induced neurotoxicity. In this study, PC12 cells were exposed to Cd alone, or after being pre-treated with Mino. Initially, the cell viability and oxidative stress were analyzed using the MTT assay and fluorimetry, respectively. Then, Cd-induced apoptosis and Mino anti-apoptotic effect were evaluated in both intrinsic and extrinsic pathways using western blot analysis. Exposing PC12 cells to Cd for 24 h decreased cell viability and increased production of reactive oxygen species in comparison with the control group. Cd (35 μM) also elevated the level of caspase-8, Bax/Bcl-2, and caspase-3 proteins in the cells. Mino pre-treatment for 2 h (100 nM) increased the number of viable cells and decreased the production of reactive oxygen species, and the level of all apoptotic markers in comparison to Cd-treated cells. Considering all the evidence, it appears that Mino holds promising antioxidant and anti-apoptotic activity and can protect cells against Cd-induced oxidative stress and prevent apoptotic cell death.






Similar content being viewed by others
Data Availability
All collected data and experiments are available in the article.
References
Flora SJS, Agrawal S (2017) Arsenic, cadmium, and lead. In: Gupta R (ed) Reproductive and developmental toxicology. Elsevier, Hopkinsville, pp 537–566
Kumar A, Pandey R, Siddiqi NJ, Sharma B (2019) Oxidative stress biomarkers of cadmium toxicity in mammalian systems and their distinct ameliorative strategy. J Appl Biotechnol 6:126–135. https://doi.org/10.15406/jabb.2019.06.00184
Zhang H, Reynolds M (2019) Cadmium exposure in living organisms: a short review. Sci Total Env 678:761–767. https://doi.org/10.1016/j.scitotenv.2019.04.395
Järup L, Åkesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238:201–208
Nordberg GF (2004) Cadmium and health in the 21st Century – historical remarks and trends for the future. Biometals 17:485–489. https://doi.org/10.1023/B:BIOM.0000045726.75367.85
Fatima G, Raza AM, Hadi N et al (2019) Cadmium in human diseases: it’s more than just a mere metal. Indian J Clin Biochem 34:371–378
Rahimzadeh MR, Rahimzadeh MR, Kazemi S, Moghadamnia AA (2017) Cadmium toxicity and treatment: An update. Casp J Intern Med 8:135–145. https://doi.org/10.22088/cjim.8.3.135
El-Kott AF, Alshehri AS, Khalifa HS et al (2020) Cadmium chloride induces memory deficits and hippocampal damage by activating the JNK/p66Shc/NADPH oxidase axis. Int J Toxicol 39:477–490. https://doi.org/10.1177/1091581820930651
Branca JJV, Morucci G, Pacini A (2018) Cadmium-induced neurotoxicity: Still much ado. Neural Regen Res 13:1879–1882
Malin AJ, Wright RO (2018) The developmental neurotoxicity of cadmium. Handbook of developmental neurotoxicology, 2nd edn. Elsevier, New York, pp 407–412
Al Olayan EM, Aloufi AS, AlAmri OD, et al (2020) Protocatechuic acid mitigates cadmium-induced neurotoxicity in rats: role of oxidative stress, inflammation and apoptosis. Sci Total Env 723:.https://doi.org/10.1016/j.scitotenv.2020.137969
Khan A, Ikram M, Muhammad T et al (2019) Caffeine modulates cadmium-induced oxidative stress, neuroinflammation, and cognitive impairments by regulating Nrf-2/HO-1 in vivo and in vitro. J Clin Med 8:680
Tabeshpour J, Mehri S, Shaebani Behbahani F, Hosseinzadeh H (2018) Protective effects of Vitis vinifera (grapes) and one of its biologically active constituents, resveratrol, against natural and chemical toxicities: a comprehensive review. Phytother Res 32:2164–2190. https://doi.org/10.1002/ptr.6168
Omidkhoda SF, Razavi BM, Hosseinzadeh H (2019) Protective effects of Ginkgo biloba L. against natural toxins, chemical toxicities, and radiation: a comprehensive review. Phytother Res 33:2821–2840. https://doi.org/10.1002/ptr.6469
Tavakkoli A, Ahmadi A, Razavi BM, Hosseinzadeh H (2017) Black Seed (Nigella Sativa) and its constituent Thymoquinone as an antidote or a protective agent against natural or chemical toxicities. Iran J Pharm Res IJPR 16:2–23
Alavi MS, Fanoudi S, Ghasemzadeh Rahbardar M, et al (2020) An updated review of protective effects of rosemary and its active constituents against natural and chemical toxicities. Phyther Res ptr.6894. https://doi.org/10.1002/ptr.6894
Metz LM, Li DKB, Traboulsee AL et al (2017) Trial of minocycline in a clinically isolated syndrome of Multiple Sclerosis. N Engl J Med 376:2122–2133. https://doi.org/10.1056/nejmoa1608889
Gunn GB, Mendoza TR, Garden AS et al (2020) Minocycline for symptom reduction during radiation therapy for head and neck cancer: a randomized clinical trial. Support Care Cancer 28:261–269. https://doi.org/10.1007/s00520-019-04791-4
Bonelli RM, Hödl AK, Hofmann P, Kapfhammer H-P (2004) Neuroprotection in Huntington’s disease: a 2-year study on minocycline. Int Clin Psychopharmacol 19:337–342
Verma DK, Singh DK, Gupta S et al (2018) Minocycline diminishes the rotenone induced neurotoxicity and glial activation via suppression of apoptosis, nitrite levels and oxidative stress. Neurotoxicology 65:9–21. https://doi.org/10.1016/j.neuro.2018.01.006
Popovic N, Schubart A, Goetz BD et al (2002) Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann Neurol 51:215–223. https://doi.org/10.1002/ana.10092
Choi Y, Kim H-S, Shin KY et al (2007) Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer’s Disease models. Neuropsychopharmacology 32:2393–2404. https://doi.org/10.1038/sj.npp.1301377
Du Y, Ma Z, Lin S et al (2001) Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci U S A 98:14669–14674. https://doi.org/10.1073/pnas.251341998
O’Dell JR, Blakely KW, Mallek JA et al (2001) Treatment of early seropositive rheumatoid arthritis: a two-year, double-blind comparison of minocycline and hydroxychloroquine. Arthritis Rheum 44:2235–2241
Lian HD, Ren B, Gao XW (2011) Effects of minocycline on expression of bcl-2, bax in early retinal neuropathy of diabetes in rats. Int J Ophthalmol 4:162–164. https://doi.org/10.3980/j.issn.2222-3959.2011.02.10
Zhou YQ, Liu DQ, Chen SP et al (2018) Minocycline as a promising therapeutic strategy for chronic pain. Pharmacol Res 134:305–310
Bastos LFS, Prazeres JDM, Godin AM et al (2013) Sex-independent suppression of experimental inflammatory pain by minocycline in two mouse strains. Neurosci Lett 553:110–114. https://doi.org/10.1016/j.neulet.2013.08.026
Cho I-H, Chung YM, Park C-K et al (2006) Systemic administration of minocycline inhibits formalin-induced inflammatory pain in rat. Brain Res 1072:208–214. https://doi.org/10.1016/j.brainres.2005.12.039
Zhang G, Yu L, Chen Z-Y et al (2016) Activation of corticotropin-releasing factor neurons and microglia in paraventricular nucleus precipitates visceral hypersensitivity induced by colorectal distension in rats. Brain Behav Immun 55:93–104. https://doi.org/10.1016/j.bbi.2015.12.022
Kumar V, Singh BK, Chauhan AK et al (2016) Minocycline rescues from zinc-induced nigrostriatal dopaminergic neurodegeneration: biochemical and molecular iInterventions. Mol Neurobiol 53:2761–2777. https://doi.org/10.1007/s12035-015-9137-y
Zhang L, Xiao H, Yu X, Deng Y (2020) Minocycline attenuates neurological impairment and regulates iron metabolism in a rat model of traumatic brain injury. Arch Biochem Biophys 682:108302. https://doi.org/10.1016/j.abb.2020.108302
Guo J, Chen Q, Tang J et al (2015) Minocycline-induced attenuation of iron overload and brain injury after experimental germinal matrix hemorrhage. Brain Res 1594:115–124. https://doi.org/10.1016/j.brainres.2014.10.046
Zhao F, Wang J, Lu H et al (2020) Neuroprotection by walnut-derived peptides through autophagy promotion via Akt/mTOR signaling pathway against oxidative stress in PC12 cells. J Agric Food Chem 68:3638–3648. https://doi.org/10.1021/acs.jafc.9b08252
Peng S, Hou Y, Yao J, Fang J (2019) Neuroprotection of mangiferin against oxidative damage via arousing Nrf2 signaling pathway in PC12 cells. BioFactors 45:381–392. https://doi.org/10.1002/biof.1488
Mehri S, Abnous K, Mousavi SH et al (2012) Neuroprotective effect of crocin on acrylamide-induced cytotoxicity in PC12 cells. Cell Mol Neurobiol 32:227–235. https://doi.org/10.1007/s10571-011-9752-8
Ganguly K, Levänen B, Palmberg L et al (2018) Cadmium in tobacco smokers: a neglected link to lung disease? Eur Respir Rev 27:1–8. https://doi.org/10.1183/16000617.0122-2017
Cankaya S, Cankaya B, Kilic U et al (2019) The therapeutic role of minocycline in Parkinson’s disease. Drugs Context 8:1–14. https://doi.org/10.7573/dic.212553
Banik S, Akter M, Corpus Bondad SE et al (2019) Carvacrol inhibits cadmium toxicity through combating against caspase dependent/independent apoptosis in PC12 cells. Food Chem Toxicol 134:110835. https://doi.org/10.1016/j.fct.2019.110835
Binte Hossain KF, Rahman MM, Sikder MT et al (2018) Inhibitory effects of selenium on cadmium-induced cytotoxicity in PC12 cells via regulating oxidative stress and apoptosis. Food Chem Toxicol 114:180–189. https://doi.org/10.1016/j.fct.2018.02.034
Ben P, Zhang Z, Xuan C et al (2015) Protective effect of l-Theanine on cadmium-induced apoptosis in PC12 cells by inhibiting the mitochondria-mediated pathway. Neurochem Res 40:1661–1670. https://doi.org/10.1007/s11064-015-1648-4
López E, Arce C, Oset-Gasque MJ et al (2006) Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radic Biol Med 40:940–951. https://doi.org/10.1016/j.freeradbiomed.2005.10.062
Chen L, Xu B, Liu L et al (2011) Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Rad Biol Medi 50:624–632. https://doi.org/10.1016/j.freeradbiomed.2010.12.032
Jiang B-P, Le L, Xu L-J, Xiao P-G (2014) Minocycline inhibits ICAD degradation and the NF-κB activation induced by 6-OHDA in PC12 cells. Brain Res 1586:1–11. https://doi.org/10.1016/j.brainres.2014.08.001
Algeciras-Schimnich A, Barnhart BC, Peter ME (2013) Apoptosis dependent and independent functions of caspases. In: Madame Curie Bioscience Database [Internet]. Landes Bioscience, Austin, pp 98–119
Fulda S, Debatin K-M (2006) Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25:4798–4811. https://doi.org/10.1038/sj.onc.1209608
Wajant H (2002) The Fas signaling pathway: more than a paradigm. Science 296:1635–1636. https://doi.org/10.1126/science.1071553
Ospondpant D, Phuagkhaopong S, Suknuntha K et al (2019) Cadmium induces apoptotic program imbalance and cell cycle inhibitor expression in cultured human astrocytes. Env Toxicol Pharmacol 65:53–59. https://doi.org/10.1016/j.etap.2018.12.001
Yuan Y, Zhang Y, Zhao S et al (2018) Cadmium-induced apoptosis in neuronal cells is mediated by Fas/FasL-mediated mitochondrial apoptotic signaling pathway w. Sci Rep 8:1–11. https://doi.org/10.1038/s41598-018-27106-9
Lee CY, Su CH, Tsai PK et al (2018) Cadmium nitrate-induced neuronal apoptosis is protected by N-acetyl-L-cysteine via reducing reactive oxygen species generation and mitochondria dysfunction. Biomed Pharmacother 108:448–456. https://doi.org/10.1016/j.biopha.2018.09.054
Alian NS, Khodarahmi P, Naseh V (2018) The effect of cadmium on apoptotic genes mRNA expression of Bax and Bcl-2 in small intestine of rats. Iran J Pathol 13:408–414
Chouit Z, Djellal D, Haddad S et al (2021) Potentiation of the apoptotic signaling pathway in both the striatum and hippocampus and neurobehavioral impairment in rats exposed chronically to a low−dose of cadmium. Environ Sci Pollut Res 28:3307–3317. https://doi.org/10.1007/s11356-020-10755-7
Wen S, Wang L, Zhang W, et al (2021) Induction of mitochondrial apoptosis pathway mediated through caspase-8 and c-Jun N-terminal kinase by cadmium-activated Fas in rat cortical neurons. Metallomics 13:.https://doi.org/10.1093/mtomcs/mfab042
Wang X, Zhu S, Drozda M et al (2003) Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington’s disease. Proc Natl Acad Sci U S A 100:10483–10487. https://doi.org/10.1073/pnas.1832501100
Kelly KJ, Sutton TA, Weathered N et al (2004) Minocycline inhibits apoptosis and inflammation in a rat model of ischemic renal injury. Am J Physiol Ren Physiol Ren Physiol 287:760–766. https://doi.org/10.1152/ajprenal.00050.2004
Heo K, Cho YJ, Cho KJ et al (2006) Minocycline inhibits caspase-dependent and -independent cell death pathways and is neuroprotective against hippocampal damage after treatment with kainic acid in mice. Neurosci Lett 398:195–200. https://doi.org/10.1016/j.neulet.2006.01.027
Wang B, Lin W, Zhu H (2021) Minocycline improves the recovery of nerve function and alleviates blood-brain barrier damage by inhibiting endoplasmic reticulum in traumatic brain injury mice model. Eur J Inflamm 19:205873922110108. https://doi.org/10.1177/20587392211010898
Acknowledgements
The authors are thankful to the Vice-Chancellor of Research, Mashhad University of Medical Sciences, for financial support. The results presented in this paper are part of a Pharm.D thesis.
Funding
This study was financially supported by a grant from the Vice-Chancellor of Research, Mashhad University of Medical Sciences (Grant number: 981440).
Author information
Authors and Affiliations
Contributions
MSH did the research, analyzed the data, and wrote the paper. SM supervised, designed the experiments, verified the analytical methods, and checked the whole procedure and paper. BMR designed the experiments. HH supervised, conceived the original idea, and checked the whole procedure and paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval
This article does not include any experiments on animals or human participants.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shayan, M., Mehri, S., Razavi, B.M. et al. Minocycline Protects PC12 Cells Against Cadmium-Induced Neurotoxicity by Modulating Apoptosis. Biol Trace Elem Res 201, 1946–1954 (2023). https://doi.org/10.1007/s12011-022-03305-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03305-4