Abstract
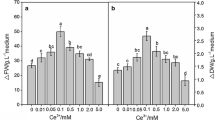
The effects of rare earth elements (REEs) not only on cell growth and flavonoid accumulation of Tetrastigma hemsleyanum suspension cells but also on the isoenzyme patterns and activities of related enzymes were studied in this paper. There were no significant differences in enhancement of flavonoid accumulation in T. hemsleyanum suspension cells among La3+, Ce3+, and Nd3+. Whereas their inductive effects on cell proliferation varied greatly. The most significant effects were achieved with 100 μM Ce3+and Nd3+. Under treatment over a 25-day culture period, the maximal biomass levels reached 1.92- and 1.74-fold and the total flavonoid contents are 1.45- and 1.49-fold, than that of control, respectively. Catalase, phenylalanine ammonia-lyase (PAL), and peroxidase (POD) activity was activated significantly when the REE concentration range from 0 to 300 μM, whereas no significant changes were found in superoxide dismutase activity. Differences of esterase isozymes under REE treatment only laid in expression level, and there were no specific bands. The expression level of some POD isozymes strengthened with increasing concentration of REEs within the range of 50–200 μM. When REE concentration was higher than 300 μM, the expression of some POD isozymes was inhibited; meanwhile, some other new POD isozymes were induced. Our results also showed REEs did not directly influence PAL activity. So, we speculated that 50–200 μM REEs could activate some of antioxidant enzymes, adjust some isozymes expression, trigger the defense responses of T. hemsleyanum suspension cells, and stimulate flavonoid accumulation by inducing PAL activity.



Similar content being viewed by others
Abbreviations
- REEs:
-
Rare earth elements
- PAL:
-
Phenylalanine ammonia-lyase
- SOD:
-
Superoxide dismutase
- NAA:
-
Naphthalene acetic acid
- EST:
-
Esterase
- FW:
-
Fresh weight
- CAT:
-
Catalase
- ROS:
-
Reactive oxygen species
- POD:
-
Peroxidase
- 6-BA:
-
6-Benzyladenine
- DW:
-
Dry weight
References
Xu CJ, Ding GQ, Fu JY et al (2008) Immunoregulatory effects of ethyl-acetate fraction of extracts from Tetrastigma hemsleyanum Diels et. Gilg on immune functions of ICR mice. Biomed Environ Sci 21(4):325–331
He FG (2010) Research progress in anticancer effect of Tetrastigma hemsleyanum Diels et Gilg and its mechanism. J Oncol 16:75–77 (in Chinese)
Peng X, Zhang J, He JY (2012) Comparison on accumulation of flavonoids in loose and compact callus suspension cell culture of Tetrastigma hemsleyanum. Chin Tradit Herb Drugs 43:577–580 (in chinese)
Karwasara VS, Jain R, Tomar P et al (2010) Elicitation as yield enhancement strategy for glycyrrhizin production by cell cultures of Abrus precatorius Linn. In Vitro Cell Dev Biol Plant 46:354–362
Chakraborty A, Chattopadhyay S (2008) Stimulation of menthol production in Mentha piperita cell culture. In Vitro Cell Dev Biol Plant 44:518–524
Karwasara VS, Dixit VK (2012) Culture medium optimization for improved puerarin production by cell suspension cultures of Pueraria tuberosa (Roxb. ex Willd.) DC. In Vitro Cell Dev Biol Plant 48:189–199
Ge F, Wang XD, Zhao B, Wang YC (2006) Effects of rare earth elements on the growth of Arnebia euchroma cells and the biosynthesis of shikonin. Plant Growth Regul 48:283–290
Yuan XF, Zhao B, Wang YC (2005) Application of rare earth elements in medicinal plant cell and tissue culture. Chin Bull Bot 22:115–120
Zhou M, Gong X, Wang Y et al (2011) Improvement of cerium of photosynthesis functions of maize under magnesium deficiency. Biol Trace Elem Res 142:760–772
Liu XQ, Ze YG, Liu C et al (2009) Effects of Ce3+ on improvement of spectral characteristics and function of chloroplasts damaged by linolenic acid in spinach. J Rare Earths 27:288–293
Huang H, Chen L, Liu XQ et al (2008) Absorption and transfer of light and photoreduction activities of spinach chloroplasts under calcium deficiency: promotion by cerium. Biol Trace Elem Res 122:157–167
Huang G, Wang L, Zhou Q (2012) Lanthanum (III) regulates the nitrogen assimilation in soybean seedlings under ultraviolet-B radiation. Biol Trace Elem Res. doi:10.1007/s12011-012-9528-0
Olivares E, Aguiar G, Colonnello G (2011) Rare earth elements in vascular plants: a review. Interciencia 36(5):331–340
Diatloff E, Smith FW, Asher CJ (2008) Effects of lanthanum and cerium on the growth and mineral nutrition of corn and mungbean. Ann Bot 101(7):971–982
d’Aquino L, Massimo M, Carboni MA (2009) Effect of some rare earth elements on the growth and lanthanide accumulation in different Trichoderma strains. Soil Biol Biochem 41:2406–2413
Boyko A, Matsuoka A, Kovalchuk I (2011) Potassium chloride and rare earth elements improve plant growth and increase the frequency of the Agrobacterium tumefaciens-mediated plant transformation. Plant Cell Rep 30:505–518
Wang CT, Shi GX, Xu QS (2005) Toxic effect of Cd2+ on Potamogeton crispus alleviated by exogenous Nd3+. J Rare Earths 23(6):752–755 (in chinese)
Liu YJ, Wang Y, Wang FB (2008) Control effect of lanthanum against plant disease. J Rare Earths 26:115–120
Osorio S, Alba R, Damasceno CM et al (2011) Systems biology of tomato fruit development: combined transcript, protein, and metabolite analysis of tomato transcription factor and ethylene receptor mutants reveals novel regulatory interactions. Plant Physiol 157(1):405–425
Bowler C, Camp WV, Montagy MV et al (1994) Superoxide dismutase in plants. Crit Rev Plant Sci 13(3):199–218
Ippolito MP, Fasciano C, d’Aquino L et al (2010) Responses of antioxidant systems after exposition to rare earths and their role in chilling stress in common duckweed (Lemna minor L.): a defensive weapon or a boomerang? Arch Environ Contam Toxicol 58:42–52
Fl Z, Tian HE, Wang ZP et al (2003) Effect of rare earth element europium on amaranthin synthesis in Amarathus caudatus seedlings. Biol Trace Elem Res 93:271–282
Zhishen JT, Mengcheng T, Jianming W (1999) Research on antioxidant activity of flavonoids from natural materials. Food Chem 64:555–559
Cipollini DF Jr (1998) The induction of soluble peroxidase activity in bean leaves by wind-induced mechanical perturbation. Am J Bot 85(11):1586–1591
Wang YS, Tian SP, Xu Y et al (2004) Changes in the activities of pro- and anti-oxidant enzymes in peach fruit inoculated with Cryptococcus laurentii or Penicillium expansum at 0 or 20°C. Postharvest Biol Technol 34:21–28
Peng X, Jin WT, Ling QZ (2012) Changes of phenolics content and related enzymatic activities in Fritillaria thunbergii Miq. and their relationships with dormancy release. Med Plant 3(6):44–47
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assay applicable to acrylamide gels. Anal Biochem 44:276–287
Mandak B, Bimova K, Pysek P (2005) Isoenzyme diversity in Reynoutria (Polygonaceae) taxa: escape from sterility by hybridization. Plant Syst Evol 253:219–230
Huang WH (2011) Biological effect of rare earth element and genetic transformation on Arnebia euehroma (Royle) Johnst. cell. Dissertation, University of Kunming Science and Technology (in Chinese)
Ozaki T, Enomoto S, Minai Y (2000) Beneficial effect of rare earth elements on the growth of Dryopteris erythrosora. J Plant Physiol 156:330–334
Gao YS, Zeng FL, Yi A (2003) Research of the entry of rare earth elements Eu3+ and La3+ into plant cell. Biol Trace Elem Res 91:253–265
Wei ZG, Hong FS, Yin M et al (2005) Structural differences between light and heavy rare earth element binding chlorophylls in naturally grown fern Dicranopteris linearis. Biol Trace Elem Res 106:279–297
Liang T, Yan BZ, Zhang S (2001) Contents and the biogeochemical characteristics of rare earth elements in wheat seeds. Biogeochemistry 54:41–49
Ouyang J, Xiaodong W, Bing Z et al (2003) Effects of rare earth elements on the growth of Cistanch deserticola cells and the production of phenylethanoid glycosides. J Biotechnol 102:129–134
Yuan XF, Wang Q, Zhao B et al (2002) Improved cell growth and total flavonoids of Saussurea medusa on solid culture medium supplemented with rare earth elements. Biotechnol Lett 24:1889–1892
Jing F, Bei W, Shan XQ et al (2007) Evaluation of bioavailability of light rare earth elements to wheat (Triticum aestivum L.)under field conditions. Geoderma 141:53–59
Wu JY, Wang CG, Mei XG (2001) Stimulation of taxol production and excretion in Taxus spp cell cultures by rare earth chemical lanthanum. J Biotechnol 85:67–73
Zhou J, Fang L, Li X et al (2012) Jasmonic acid (JA) acts as a signal molecule in LaCl3-induced baicalin synthesis in Scutellaria baicalensis seedlings. Biol Trace Elem Res 148:392–395
Liu C, Cao WQ, Lu Y et al (2009) Cerium under calcium deficiency influence on the antioxidative defense system in spinach plants. Plant and Soil 323:285–294
Ze YG, Zhou M, Luo LY et al (2009) Effects of cerium on key enzymes of carbon assimilation of spinach under magnesium deficiency. Biol Trace Elem Res 131(2):154–164
Yang GM, Sun ZG, Lv XF et al (2012) Living target of Ce(III) action on horseradish cells: proteins on/in cell membrane. Biol Trace Elem Res. doi:10.1007/s12011-012-9514-6
Guo B, Xu L, Guan ZJ et al (2012) Effect of lanthanum on rooting of in vitro regenerated shoots of Saussurea involucrata Kar. et Kir. Biol Trace Elem Res 147:334–340
Wang L, Huang X, Zhou Q (2009) Protective effect of rare earth against oxidative stress under ultraviolet-B radiation. Biol Trace Elem Res 128:82–93
Paola M, Paciolla C, d'Aquino L et al (2007) Effect of rare earth elements on growth and antioxidant metabolism in Lemna minor L. Caryologia 60:125–128
Shi P, Chen GC, Huang ZW (2005) Effects of La3+ on the active oxygen scavenging enzyme activities in cucumber seedling leaves. Russ J Plant Physiol 52:294–297
Yuan YJ, Li JC, Ge ZQ et al (2002) Superoxide anion burst and taxol production induced by Ce4+ in suspension cultures of Taxus cuspidata. J Mol Catal B: Enzym 18:251–260
Wang YL, Wang XD, Zhao B et al (2007) Reduction of hyperhydricity in the culture of Lepidium meyenii shoots by the addition of rare earth elements. Plant Growth Regul 52:151–159
Camm EL, Towers GHN (1973) Review article: phenylalanine ammonia lyase. Phytochemistry 12:961–973
Lister CE, Lancaster JE, Walker JRL (1996) Phenylalanine ammonia-lyase (PAL) activity and its relationship to anthocyanin and flavonoid levels in New Zealand-grown apple cultivars. J Am Soc Hortic Sci 12(2):281–285
Creasy LL (1971) Role of phenylalanine in the biosynthesis of flavonoids and cinnamic acids in strawberry leaf disks. Phytochemistry 10(11):2705–2711
Eichholza I, Rohn S, Gamm A et al (2012) UV-B-mediated flavonoid synthesis in white asparagus (Asparagus offcinalis L.). Food Res Int 48:196–201
Huang J, Gu M, Lai Z et al (2010) Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol 153(4):1526–1538
Hossain Z, Mandal AK, Datta SK et al (2006) Development of NaCl tolerant strain in Chrysanthemum morifolium Ramat. through in vitro mutagenesis. Plant Biol 8(4):450–461
Muarlidharan J, John E, Channamma L et al (1996) Change in esterases in response to blast infection in fingermillet seedlings. Phytochemistry 43:1151–1155
Sahoo MR, Dasgupta M, Kole PC et al (2007) Antioxidative enzymes and isozymes analysis of taro genotypes and their implications in Phytophthora blight disease resistance. Mycopathologia 163(4):241–248
Roy S, Begum Y, Chakraborty A et al (2006) Radiation-induced phenotypic alterations in relation to isozymes and RAPD markers in Vigna radiate (L) Wilczek. Int J Radiat Biol 82(11):823–832
Bogdanovic J, Milosavic N, Prodanovic R et al (2007) Variability of antioxidant enzyme activity and isoenzyme profile in needles of Serbian spruce (Picea omorika (Panc.) Purkinye). Biochem Syst Ecol 35:263–273
Acknowledgments
This work was supported by the Natural Science Foundation of Ningbo City (grant no. 2012A610179) and the New Shoot Talents Program of Zhejiang Province (grant no. 2010R433006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xin, P., Shuang-Lin, Z., Jun-Yao, H. et al. Influence of Rare Earth Elements on Metabolism and Related Enzyme Activity and Isozyme Expression in Tetrastigma hemsleyanum Cell Suspension Cultures. Biol Trace Elem Res 152, 82–90 (2013). https://doi.org/10.1007/s12011-013-9600-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-013-9600-4