Abstract
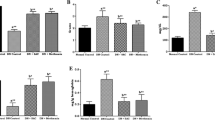
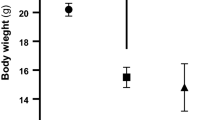
Zinc is an element that under physiological conditions preferentially binds to and is a potent inducer of metallothionein under physiological conditions. The present study was conducted to explore whether zinc supplementation morphologically and biochemically protects against diabetic nephropathy through modulation of kidney metallothionein induction and oxidative stress in streptozotocin-induced diabetic rats. Thirty-two Wistar albino male rats were equally divided into four groups. The first group was used as untreated controls and the second group was supplemented with 30 mg/kg/day zinc as zinc sulfate. The third group was treated with streptozotocin to induce diabetes and the fourth group was treated with streptozotocin and supplemented with zinc as described for group 2. The blood glucose and micro-albuminuria levels, body and kidney weights were measured during the 42-day experimental period. At the end of the experiment, the kidneys were removed from all animals from the four groups. Diabetes resulted in degenerative kidney morphological changes. The metallothionein immunoreactivity level was lower and the kidney lipid peroxidation levels were higher in the diabetes group than in the controls. The metallothionein immunoreactivity levels were higher in the tubules of the zinc-supplemented diabetic rats as compared to the non-supplemented diabetic group. The zinc and metallothionein concentrations in kidney tissue were higher in the supplemented diabetic group compared to the non-supplemented diabetes group. The activity of glutathione peroxidase did not change in any of the four groups. In conclusion, the present study shows that zinc has a protective effect against diabetic damage of kidney tissue through stimulation of metallothionein synthesis and regulation of the oxidative stress.




Similar content being viewed by others
References
Nazıroğlu M, Dikici DM, Dursun S (2012) Role of oxidative stress and Ca(2+) signaling on molecular pathways of neuropathic pain in diabetes: focus on TRP channels. Neurochem Res. (ın press)
Özkaya D, Nazıroğlu M, Armağan A, Demirel A, Köroglu BK, Çolakoğlu N, Kükner A, Sönmez TT (2011) Dietary vitamin C and E modulates oxidative stress induced-kidney and lens injury in diabetic aged male rats through modulating glucose homeostasis and antioxidant systems. Cell Biochem Funct 29:287–293
Salgueiro MJ, Krebs N, Zubillaga MB, Weill R, Postaire E, Lysionek AE, Caro RA, De Paoli T, Hager A, Boccio J (2001) Zinc and diabetes mellitus: is there a need of zinc supplementation in diabetes mellitus patients? Biol Trace Elem Res 81:215–228
Batista MN, Cuppari L, de Fatima Campos Pedrosa L, Almeida MG, de Almeida JB, de Medeiros AC, Canziani ME (2006) Effect of end-stage renal disease and diabetes on zinc and copper status. Biol Trace Elem Res 112:1–12
Özcelik D, Tuncdemir M, Özturk M, Uzun H (2011) Evaluation of trace elements and oxidative stress levels in the liver and kidney of streptozotocin-induced experimental diabetic rat model. Gen Physiol Biophys 30:356–363
Powell SR (2000) The antioxidant properties of zinc. J Nutr 130:1447S–1454S
Brandao-Neto J, Silva CAB, Rezende AA, Almeida MG, Sales VSP, Marchini JS (2003) Zinc pharmacokinetics in insulin-dependent diabetes mellitus patients after oral zinc tolerance test. Nutr Res 23:141–150
Adachi Y, Yoshida J, Kodera Y, Kiss T, Jakusch T, Enyedy EA, Yoshikawa Y, Sakurai H (2006) Oral administration of a zinc complex improves type diabetes and metabolic syndromes. Biochem Biophys Res Commun 351:165–170
Alscher DM, Braun N, Biegger D, Stuelten C, Gawronski K, Murdter TE, Kuhlmann U, Fritz P (2005) Induction of metallothionein in proximal tubular cells by zinc and its potential as an endogenous antioxidant. Kidney Blood Press Res 28:127–133
Liuzzi JP, Cousins RJ (2004) Mammalian zinc transporters. Annu Rev Nutr 24:151–172
Zhou Z, SunX KYJ (2002) Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp Biol Med 227:214–222
Cai L (2004) Metallothionein as an adaptive protein prevents diabetes and its toxicity. Nonlinearity Biol-Toxicol-Med 2:89–103
Wang J, Song Y, Elsherif L, Song Z, Zhou G, Prabhu SD, Saari JT, Cai L (2006) Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation 113:544–554
Tunçdemir M, Ozturk M (2008) The effects of ACE inhibitor and angiotensin receptor blocker on clusterin and apoptosis in the kidney tissue of streptozotocin-diabetic rats. J Mol Histol 39:605–616
Placer ZA, Cushman L, Johnson BC (1966) Estimation of products of lipid peroxidation (malonyl dialdehyde) in biological fluids. Anal Biochem 16:359–364
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin-Phenol reagent. J Biol Chem 193:265–275
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71:952–958
Clegg MS, Keen CL, Lönnerdal B, Hurley LS (1981) Influence of ashing techniques in the analyses of trace elements in animal tissue. Biol Trace Elem Res 3:107–115
Ravi K, Ramachandran B, Subramanian S (2004) Protective effect of Eugenia jambolana seed kernel on tissue antioxidants in streptozotocin induced diabetic rats. Biol Pharm Bull 27:1212–1217
Orhan N, Berkkan A, Deliorman Orhan D, Aslan M, Ergun F (2011) Effects of Juniperus oxycedrus ssp. oxycedrus on tissue lipid peroxidation, trace elements (Cu, Zn, Fe) and blood glucose levels in experimental diabetes. J Ethnopharmacol 133:759–764
Ziyadeh FN, Goldfarb S (1991) The renal tubulointerstitium in diabetes mellitus. Kidney Int 39:464–475
Kelly DJ, Cox AJ, Tolcos M, Cooper ME, Wilkinson-Berka JL, Gilbert RE (2002) Attenuation of tubular apoptosis by blockade of the renin–angiotensin system in diabetic Ren-2 rats. Kidney Int 61:31–39
Sanai T, Sobka T, Johnson T, el-Essawy M, Muchaneta-Kubara EC, Ben Gharbia O, el Oldroyd S, Nahas AM (2000) Expression of cytoskeletal proteins during the course of experimental diabetic nephropathy. Diabetologia 43:91–100
Hill C, Flyvbjerg A, Gronbeak H, Petrik J, Hill DJ, Thomas CR, Sheppard MC, Logan A (2000) The renal expression of transforming growth factor-b isoforms and their receptors in acute and chronic experimental diabetes in rats. Endocrinology 141:1196–1208
Kovacic P, Somanathan R (2008) Unifying mechanism for eye toxicity: electron transfer, reactive oxygen species, antioxidant benefits, cell signaling and cell membranes. Cell Membr Free Radic Res 2:56–69
Nazıroğlu M (2007) New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32:1990–2001
Kakkar R, Karla J, Mantha SV, Prasad K (1995) Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol Cell Biochem 151:113–119
Kutlu M, Nazıroğlu M, Simşek H, Yilmaz T, Sahap KA (2005) Moderate exercise combined with dietary vitamins C and E counteracts oxidative stress in the kidney and lens of streptozotocin-induced diabetic-rat. Int J Vitam Nutr Res 75:71–80
Simşek M, Nazıroğlu M, Erdinç A (2005) Moderate exercise with a dietary vitamin C and E combination protects against streptozotocin-induced oxidative damage to the kidney and lens in pregnant rats. Exp Clin Endocrinol Diabetes 113:53–59
Szczurek EI, Bjornsson CS, Noto AD, Taylor CG (2009) Renal metallothionein responds rapidly and site specifically to zinc repletion in growing rats. J Trace Elem Med Biol 23:176–182
Li X, Cai L, Feng W (2007) Diabetes and metallothionein. Mini Rev Med Chem 7:761–768
Dinesh Kumar S, Vijaya M, Perumal Samy R, Thameem Dheen S, Ren M, Watt F, James Kang Y, Bay BH, Tay SS (2012) Zinc supplementation prevents cardiomyocyte apoptosis and congenital heart defects in embryos of diabetic mice. Free Radic Biol Med. http://dx.doi.org/10.1016/j.freeradbiomed.2012.07.008
Tang Y, Yang Q, Lu J, Zhang X, Suen D, Tan Y, Jin L, Xiao J, Xie R, Rane M, Li X, Cai L (2010) Zinc supplementation partially prevents renal pathological changes in diabetic rats. J Nutr Biochem 21:237–246
Zou MH, Shi C, Cohen RA (2002) Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest 109:817–826
Jin T, Nordberg GF, Sehlin J, Leffler P, Wu J (1994) The susceptibility of spontaneously diabetic mice to cadmium-metallothionein nephrotoxicity. Toxicology 89:81–90
Takano H, SatohM SA, Sagai M, Yoshikawa T, Tohyama C (2000) Cytoprotection by metallothionein against gastroduodenal mucosal injury caused by ethanol in mice. Lab Invest 80:371–377
Acknowledgments
The authors thank the Scientific Research Projects Coordination Unit of Istanbul University for the support of the present study (project number 3987). DÖ formulated the present hypothesis. MÖ, ÖÇ and MT were responsible for analysis of the data. MN was responsible for writing the report. MFFA made critical revision for the manuscript. Abstract of the study was published in abstract book of 4th International Congress on Cell Membranes and Oxidative Stress: Focus on Calcium Signaling and TRP Channels, 26–29 June 2012, Isparta, Turkey.
Conflict of interest
The authors declare that there are no conflicts of interest arising from their study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Özcelik, D., Nazıroglu, M., Tunçdemir, M. et al. Zinc Supplementation Attenuates Metallothionein and Oxidative Stress Changes in Kidney of Streptozotocin-Induced Diabetic Rats. Biol Trace Elem Res 150, 342–349 (2012). https://doi.org/10.1007/s12011-012-9508-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9508-4