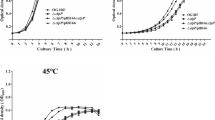
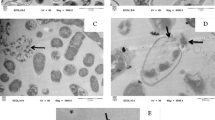
Abstract
The role of the shape of the nanostructure on the antibacterial effects of ZnO nanodisks has been investigated by detailed mass spectrometry-based proteomics along with other spectroscopic and microscopic studies on E. coli. The primary interaction study of the E. coli cells in the presence of ZnO nanodisks showed rigorous cell surface damage disrupting the cell wall/membrane components detected by microscopic and ATR-FTIR studies. Protein profiling of whole-cell extracts in the presence and absence of ZnO nanodisks identified several proteins that are upregulated and downregulated under the stress of the nanodisks. This suggests that the bacterial response to the primary stress leads to a secondary impact of ZnO nanodisk toxicity via regulation of the expression of specific proteins. Results showed that the ZnO nanodisks lead to the over-expression of peptidyl-dipeptidase Dcp, Transketolase-1, etc., which are important to maintaining the osmotic balance in the cell. The abrupt change in osmotic pressure leads to mechanical injury to the membrane, and nutritional starvation conditions, which is revealed from the expression of the key proteins involved in membrane-protein assembly, maintaining membrane integrity, cell division processes, etc. Thus, indicating a deleterious effect of ZnO nanodisk on the protective layer of E. coli. ZnO nanodisks seem to primarily affect the protective membrane layer, inducing cell death via the development of osmotic shock conditions, as one of the possible reasons for cell death. These results unravel a unique behavior of the disk-shaped ZnO nanostructure in executing lethality in E. coli, which has not been reported for other known shapes or morphologies of ZnO nanoforms.











Similar content being viewed by others
References
Sirelkhatim, A., et al. (2015). Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Letters, 7(3), 219–242. https://doi.org/10.1007/s40820-015-0040-x
Kolodziejczak-Radzimska, A., & Jesionowski, T. (2014). Zinc oxide-from synthesis to application: A review. Materials (Basel), 7(4), 2833–2881. https://doi.org/10.3390/ma7042833
Jiang, J., Pi, J., Cai, J. (2018). The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorganic Chemistry and Applications, 2018. https://doi.org/10.1155/2018/1062562
Mishra, P. K., Mishra, H., Ekielski, A., Talegaonkar, S., & Vaidya, B. (2017). Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discovery Today, 22(12), 1825–1834. https://doi.org/10.1016/j.drudis.2017.08.006
Food and Drug Administration (FDA). (2019). CFR Code of Federal Regulations Title 21., www.fda.gov, (vol. 721, no. 6, pp. 1–10), [Online]. Available: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=582.5991&SearchTerm=zinc%20oxide. Accessed 31 Mar 2021
Tong, G. X., et al. (2013). Polymorphous ZnO complex architectures: Selective synthesis, mechanism, surface area and Zn-polar plane-codetermining antibacterial activity. Journal of Materials Chemistry B, 1(4), 454–463. https://doi.org/10.1039/c2tb00132b
Patrinoiu, G., et al. (2019). Eco-friendly synthesized spherical ZnO materials: Effect of the core-shell to solid morphology transition on antimicrobial activity. Materials Science and Engineering: C, 97(November 2018), 438–450. https://doi.org/10.1016/j.msec.2018.12.063
Cai, Q., et al. (2016). Insight into biological effects of zinc oxide nanoflowers on bacteria: Why morphology matters. ACS Applied Materials & Interfaces, 8(16), 10109–10120. https://doi.org/10.1021/acsami.5b11573
Khan, M. F., et al. (2016). Sol-gel synthesis of thorn-like ZnO nanoparticles endorsing mechanical stirring effect and their antimicrobial activities: Potential role as nano-Antibiotics. Science and Reports, 6(March), 1–12. https://doi.org/10.1038/srep27689
Wang, X., Yang, F., Yang, X. (2007) A study on the antibacterial activity of one-dimensional ZnO nanowire arrays : effects of the orientation and plane surface. 4419–4421. https://doi.org/10.1039/b708662h
El-Nahas, S., El-sadek, M. S. A., Salman, H. M., & Elkady, M. M. (2021). Controlled morphological and physical properties of ZnO nanostructures synthesized by domestic microwave route. Materials Chemistry and Physics, 258(July 2020), 123885. https://doi.org/10.1016/j.matchemphys.2020.123885
Jones, N., Ray, B., Ranjit, K. T., & Manna, A. C. (2008). Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiology Letters, 279(1), 71–76. https://doi.org/10.1111/j.1574-6968.2007.01012.x
Kadiyala, U., Turali-Emre, E. S., Bahng, J. H., Kotov, N. A., & Scott Vanepps, J. (2018). Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant: Staphylococcus aureus (MRSA). Nanoscale, 10(10), 4927–4939. https://doi.org/10.1039/c7nr08499d
Khezerlou, A., Alizadeh-Sani, M., Azizi-Lalabadi, M., & Ehsani, A. (2018). Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microbial Pathogenesis, 123(August), 505–526. https://doi.org/10.1016/j.micpath.2018.08.008
Tiwari, V., Mishra, N., Gadani, K., Solanki, P. S., Shah, N. A., & Tiwari, M. (2018). Mechanism of anti-bacterial activity of zinc oxide nanoparticle against Carbapenem-Resistant Acinetobacter baumannii. Frontiers in Microbiology, 9(JUN), 1–10. https://doi.org/10.3389/fmicb.2018.01218
Xie, Y., He, Y., Irwin, P. L., Jin, T., & Shi, X. (2011). Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Applied and Environment Microbiology, 77(7), 2325–2331. https://doi.org/10.1128/AEM.02149-10
Zhang, L., Zhao, J., Zheng, J., Li, L., & Zhu, Z. (2011). Hydrothermal synthesis of hierarchical nanoparticle-decorated ZnO microdisks and the structure-enhanced acetylene sensing properties at high temperatures. Sensors and Actuators B: Chemical, 158(1), 144–150. https://doi.org/10.1016/j.snb.2011.05.057
Li, Z., Pan, W., Zhang, D., & Zhan, J. (2010). Morphology-dependent gas-sensing properties of ZnO nanostructures for chlorophenol. Chemistry - An Asian Journal, 5(8), 1854–1859. https://doi.org/10.1002/asia.201000036
Bin Jin, B., Wang, Y. F., & Zeng, J. H. (2016). Performance enhancement in titania based quantum dot sensitized solar cells through incorporation of disc shaped ZnO nanoparticles into photoanode. Chemical Physics Letters, 660, 76–80. https://doi.org/10.1016/j.cplett.2016.08.009
Hingorani, S., Pillai, V., Kumar, P., Multani, M. S., & Shah, D. O. (1993). Microemulsion mediated synthesis of zinc-oxide nanoparticles for varistor studies. Materials Research Bulletin, 28(12), 1303–1310.
Gold, K., Slay, B., Knackstedt, M., & Gaharwar, A. K. (2018). Antimicrobial activity of metal and metal-oxide based nanoparticles. Advances in Therapy, 1(3), 1700033. https://doi.org/10.1002/adtp.201700033
Djurišić, A. B., et al. (2015). Toxicity of metal oxide nanoparticles: Mechanisms, characterization, and avoiding experimental artefacts. Small (Weinheim an der Bergstrasse, Germany), 11(1), 26–44. https://doi.org/10.1002/smll.201303947
Lakshmi Prasanna, V., & Vijayaraghavan, R. (2015). Insight into the mechanism of antibacterial activity of ZnO: Surface defects mediated reactive oxygen species even in the dark. Langmuir, 31(33), 9155–9162. https://doi.org/10.1021/acs.langmuir.5b02266
Appierot, G., et al. (2009). Enhanced antibacterial actiwity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Advanced Functional Materials, 19(6), 842–852. https://doi.org/10.1002/adfm.200801081
Leung, Y. H., et al. (2016). Toxicity of ZnO and TiO2 to Escherichia coli cells. Science and Reports, 6(October), 1–13. https://doi.org/10.1038/srep35243
Ramalingam, B., Parandhaman, T., & Das, S. K. (2016). Antibacterial effects of biosynthesized silver nanoparticles on surface ultrastructure and nanomechanical properties of gram-negative bacteria viz. Escherichia coli and Pseudomonas aeruginosa. ACS Applied Materials and Interfaces, 8(7), 4963–4976. https://doi.org/10.1021/acsami.6b00161
Cui, Y., Zhao, Y., Tian, Y., Zhang, W., Lü, X., & Jiang, X. (2012). The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials, 33(7), 2327–2333. https://doi.org/10.1016/j.biomaterials.2011.11.057
Planchon, M., Leger, T., Spalla, O., Huber, G., & Ferrari, R. (2017). Metabolomic and proteomic investigations of impacts of titanium dioxide nanoparticles on Escherichia coli. PLoS ONE, 12(6), 1–23. https://doi.org/10.1371/journal.pone.0178437
Lee, J., Easteal, A. J., Pal, U., & Bhattacharyya, D. (2009). Evolution of ZnO nanostructures in sol-gel synthesis. Current Applied Physics, 9(4), 792–796. https://doi.org/10.1016/j.cap.2008.07.018
Coico, R. (2005) Gram staining basic protocol commonly used techniques. Current Protocols in Microbiology, (1), A–3C. https://doi.org/10.1002/9780471729259.mca03cs00
Kumar, A., Pandey, A. K., Singh, S. S., Shanker, R., & Dhawan, A. (2011). Engineered ZnO and TiO 2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radical Biology & Medicine, 51(10), 1872–1881. https://doi.org/10.1016/j.freeradbiomed.2011.08.025
Shevchenko, A., Tomas, H., Havliš, J., Olsen, J. V., & Mann, M. (2007). In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nature Protocols, 1(6), 2856–2860. https://doi.org/10.1038/nprot.2006.468
Holder, C. F., & Schaak, R. E. (2019). Tutorial on powder X-ray diffraction for characterizing nanoscale materials. ACS Nano, 13(7), 7359–7365. https://doi.org/10.1021/acsnano.9b05157
Zhang, X., Shi, H., Liu, E., Hu, X., Zhang, K., & Fan, J. (2021). Preparation of polycrystalline ZnO nanoparticles loaded onto graphene oxide and their antibacterial properties. Materials Today Communications, 28(June), 102531. https://doi.org/10.1016/j.mtcomm.2021.102531
Jin, D. H., Kim, D., Seo, Y., Park, H., & Huh, Y. D. (2014). Morphology-controlled synthesis of ZnO crystals with twinned structures and the morphology dependence of their antibacterial activities. Materials Letters, 115, 205–207. https://doi.org/10.1016/j.matlet.2013.10.056
Li, W. J., Shi, E. W., Zhong, W. Z., & Yin, Z. W. (1999). Growth mechanism and growth habit of oxide crystals. Journal of Crystal Growth, 203(1), 186–196. https://doi.org/10.1016/S0022-0248(99)00076-7
Cho, S., Jung, S.-H., & Lee, K.-H. (2008). Morphology-controlled growth of ZnO nanostructures using microwave irradiation: From basic to complex structures. Journal of Physical Chemistry C, 112(33), 12769–12776. https://doi.org/10.1021/jp803783s
Cho, S., Jang, J., Jung, S., Lee, B. R., Oh, E. and Lee, K. (2009). Precursor effects of citric acid and citrates on ZnO crystal formation precursor effects of citric acid and citrates on ZnO crystal formation, (27). 3825–3831. https://doi.org/10.1021/la804009g
Zhang, H., Yang, D., Li, D., Ma, X., Li, S., & Que, D. (2005). Controllable growth of ZnO microcrystals by a capping-molecule-assisted hydrothermal process. Crystal Growth & Design, 5(2), 547–550. https://doi.org/10.1021/cg049727f
Tian, Z. R., et al. (2003). Complex and oriented ZnO nanostructures. Nature Materials, 2(12), 821–826. https://doi.org/10.1038/nmat1014
Das, S., Dutta, K., & Pramanik, A. (2013). Morphology control of ZnO with citrate: A time and concentration dependent mechanistic insight. CrystEngComm, 15(32), 6349–6358. https://doi.org/10.1039/c3ce40822a
Coates, J. (2000) Interpretation of infrared spectra, a practical approach. Encyclopedia of Analytical Chemistry. © John Wiley& Sons Ltd, Chichester, 2000, pp. 10815–10837. https://doi.org/10.1097/00010694-197107000-00005
Yogamalar, N. R., Srinivasan, R., & Bose, A. C. (2009). Multi-capping agents in size confinement of ZnO nanostructured particles. Optical Materials, 31(11), 1570–1574. https://doi.org/10.1016/j.optmat.2009.03.002
Xiong, G., Pal, U., Serrano, J. G., Ucer, K. B., & Williams, R. T. (2006). Photoluminescence and FTIR study of ZnO nanoparticles: The impurity and defect perspective. Physica status solidi c, 3(10), 3577–3581. https://doi.org/10.1002/pssc.200672164
Javed, R., Usman, M., Tabassum, S., & Zia, M. (2016). Effect of capping agents: Structural, optical and biological properties of ZnO nanoparticles. Applied Surface Science, 386, 319–326. https://doi.org/10.1016/j.apsusc.2016.06.042
Mallakpour, S., & Nouruzi, N. (2017). Effects of citric acid-functionalized ZnO nanoparticles on the structural, mechanical, thermal and optical properties of polycaprolactone nanocomposite films. Materials Chemistry and Physics, 197, 129–137. https://doi.org/10.1016/j.matchemphys.2017.05.023
Čepin, M., Hribar, G., Caserman, S., & Orel, Z. C. (2015). Morphological impact of zinc oxide particles on the antibacterial activity and human epithelia toxicity. Materials Science and Engineering C, 52, 204–211. https://doi.org/10.1016/j.msec.2015.03.053
Wahab, R., et al. (2007). Low temperature solution synthesis and characterization of ZnO nano-flowers. Materials Research Bulletin, 42(9), 1640–1648. https://doi.org/10.1016/j.materresbull.2006.11.035
Khorsand Zak, A., Razali, R., Abd Majid, W. H., & Darroudi, M. (2011). Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. International Journal of Nanomedicine, 6(1), 1399–1403. https://doi.org/10.2147/ijn.s19693
Sharma, P. K., Pandey, A. C., Zolnierkiewicz, G., Guskos, N., & Rudowicz, C. (2009). Relationship between oxygen defects and the photoluminescence property of ZnO nanoparticles: A spectroscopic view. Journal of Applied Physics, 106(9), 1–6. https://doi.org/10.1063/1.3256000
Davis, K., Yarbrough, R., Froeschle, M., White, J., & Rathnayake, H. (2019). Band gap engineered zinc oxide nanostructures: Via a sol-gel synthesis of solvent driven shape-controlled crystal growth. RSC Advances, 9(26), 14638–14648. https://doi.org/10.1039/c9ra02091h
Bahnemann, D. W., Kormann, C., & Hoffmann, M. R. (1987). Preparation and characterization of quantum size zinc oxide: A detailed spectroscopic study. Journal of Physical Chemistry, 91(14), 3789–3798. https://doi.org/10.1021/j100298a015
Monticone, S., Tufeu, R., & Kanaev, A. V. (1998). Complex nature of the UV and visible fluorescence of colloidal ZnO nanoparticles. The Journal of Physical Chemistry B, 102(16), 2854–2862. https://doi.org/10.1021/jp973425p
Patrinoiu, G., Calderón-Moreno, J. M., Chifiriuc, C. M., Saviuc, C., Birjega, R., & Carp, O. (2016). Tunable ZnO spheres with high anti-biofilm and antibacterial activity via a simple green hydrothermal route. Journal of Colloid and Interface Science, 462, 64–74. https://doi.org/10.1016/j.jcis.2015.09.059
Chen, C. C., Liu, P., & Lu, C. H. (2008). Synthesis and characterization of nano-sized ZnO powders by direct precipitation method. Chemical Engineering Journal, 144(3), 509–513. https://doi.org/10.1016/j.cej.2008.07.047
Bhattacharjee, S. (2016). DLS and zeta potential - What they are and what they are not? Journal of Controlled Release, 235, 337–351. https://doi.org/10.1016/j.jconrel.2016.06.017
Zhao, X., Li, M., Lou, X. (2013) Sol – gel assisted hydrothermal synthesis of ZnO microstructures : Morphology control and photocatalytic activity. Advanced Powder Technology. 2–8. https://doi.org/10.1016/j.apt.2013.06.004
Kaper, J. B., Nataro, J. P., Mobley, H. L. T. (2004). Pathogenic Escherichia coli, 2(February). https://doi.org/10.1038/nrmicro818
Kumar, R., Umar, A., Kumar, G., & Nalwa, H. S. (2017). Antimicrobial properties of ZnO nanomaterials: A review. Ceramics International, 43(5), 3940–3961. https://doi.org/10.1016/j.ceramint.2016.12.062
Ramani, M., Ponnusamy, S., Muthamizhchelvan, C., Cullen, J., Krishnamurthy, S., & Marsili, E. (2013). Morphology-directed synthesis of ZnO nanostructures and their antibacterial activity. Colloids Surfaces B Biointerfaces, 105, 24–30. https://doi.org/10.1016/j.colsurfb.2012.12.056
Wang, Y. W., et al. (2014). Superior antibacterial activity of zinc oxide/graphene oxide composites originating from high zinc concentration localized around bacteria. ACS Applied Materials & Interfaces, 6(4), 2791–2798. https://doi.org/10.1021/am4053317
Hartmann, M., Berditsch, M., Hawecker, J., Ardakani, M. F., Gerthsen, D., & Ulrich, A. S. (2010). Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrobial Agents and Chemotherapy, 54(8), 3132–3142. https://doi.org/10.1128/AAC.00124-10
Hajipour, M. J., et al. (2012). Antibacterial properties of nanoparticles. Trends in Biotechnology, 30(10), 499–511. https://doi.org/10.1016/j.tibtech.2012.06.004
Mendes, C. R., et al. (2022). Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Science and Reports, 12(1), 1–10. https://doi.org/10.1038/s41598-022-06657-y
Ann, L. C., et al. (2014). Antibacterial responses of zinc oxide structures against Staphylococcus aureus, Pseudomonas aeruginosa and Streptococcus pyogenes. Ceramics International, 40(2), 2993–3001. https://doi.org/10.1016/j.ceramint.2013.10.008
Naumann, D. (2000). Infrared spectroscopy in microbiology. Encyclopedia of Analytical Chemistry. © John Wiley& Sons Ltd, Chichester, 2000, pp. 102–131. https://doi.org/10.1002/9780470027318.a0117.pub2
Faghihzadeh, F., Anaya, N. M., Schifman, L. A., & Oyanedel-Craver, V. (2016). Fourier transform infrared spectroscopy to assess molecular-level changes in microorganisms exposed to nanoparticles. Nanotechnology for Environmental Engineering, 1(1), 1–16. https://doi.org/10.1007/s41204-016-0001-8
Jiang, W., Yang, K., Vachet, R. W., & Xing, B. (2010). Interaction between oxide nanoparticles and biomolecules of the bacterial cell envelope as examined by infrared spectroscopy. Langmuir, 26(23), 18071–18077. https://doi.org/10.1021/la103738e
Kiwi, J., & Nadtochenko, V. (2005). Evidence for the mechanism of photocatalytic degradation of the bacterial wall membrane at the TiO2 interface by ATR-FTIR and laser kinetic spectroscopy. Langmuir, 21(10), 4631–4641. https://doi.org/10.1021/la0469831
Chakraborti, S., Mandal, A. K., Sarwar, S., Singh, P., Chakraborty, R., & Chakrabarti, P. (2014). Bactericidal effect of polyethyleneimine capped ZnO nanoparticles on multiple antibiotic resistant bacteria harboring genes of high-pathogenicity island. Colloids Surfaces B Biointerfaces, 121(September 2018), 44–53. https://doi.org/10.1016/j.colsurfb.2014.03.044
Venkatasubbu, G. D., Baskar, R., Anusuya, T., Seshan, C. A., & Chelliah, R. (2016). Toxicity mechanism of titanium dioxide and zinc oxide nanoparticles against food pathogens. Colloids Surfaces B Biointerfaces, 148(April 2019), 600–606. https://doi.org/10.1016/j.colsurfb.2016.09.042
Dwyer, D. J., Camacho, D. M., Kohanski, M. A., Callura, J. M., & Collins, J. J. (2012). Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis. Molecular Cell, 46(5), 561–572. https://doi.org/10.1016/j.molcel.2012.04.027
Henrich, B., Becker, S., Schroeder, U., & Plapp, R. (1993). dcp gene of Escherichia coli: Cloning, sequencing, transcript mapping, and characterization of the gene product. Journal of Bacteriology, 175(22), 7290–7300. https://doi.org/10.1128/jb.175.22.7290-7300.1993
Deutch, C. E., & Soffer, R. L. (1978). Escherichia coli mutants defective in dipeptidyl carboxypeptidase. Proceedings of the National Academy of Sciences, 75(12), 5998–6001. https://doi.org/10.1073/pnas.75.12.5998
Kowalska, E., Kujda, M., Wolak, N., & Kozik, A. (2012). Altered expression and activities of enzymes involved in thiamine diphosphate biosynthesis in Saccharomyces cerevisiae under oxidative and osmotic stress. FEMS Yeast Research, 12(5), 534–546. https://doi.org/10.1111/j.1567-1364.2012.00804.x
Juhnke, H., Krems, B., Kötter, P., & Entian, K. D. (1996). Mutants that show increased sensitivity to hydrogen peroxide reveal an important role for the pentose phosphate pathway in protection of yeast against oxidative stress. Molecular and General Genetics, 252(4), 456–464. https://doi.org/10.1007/BF02173011
Styrvold, O. B., & Strom, A. R. (1991). Synthesis, accumulation, and excretion of trehalose in osmotically stressed Escherichia coli K-12 strains: Influence of amber suppressors and function of the periplasmic trehalase. Journal of Bacteriology, 173(3), 1187–1192. https://doi.org/10.1128/jb.173.3.1187-1192.1991
Boos, W., Ehmann, U., Bremer, E., Middendorf, A., & Postma, P. (1987). Trehalase of Escherichia coli. Mapping and cloning of its structural gene and identification of the enzyme as a periplasmic protein induced under high osmolarity growth conditions. Journal of Biological Chemistry, 262(27), 13212–13218.
Strom, A. R., & Kaasen, I. (1993). Trehalose metabolism in Escherichia coli: Stress protection and stress regulation of gene expression. Molecular Microbiology, 8(2), 205–210. https://doi.org/10.1111/j.1365-2958.1993.tb01564.x
Lequette, Y., Ödberg-Ferragut, C., Bohin, J. P., & Lacroix, J. M. (2004). Identification of mdoD, an mdoG paralog which encodes a twin-arginine-dependent periplasmic protein that controls osmoregulated periplasmic glucan backbone structures. Journal of Bacteriology, 186(12), 3695–3702. https://doi.org/10.1128/JB.186.12.3695-3702.2004
Bohin, J. P. (2000). Osmoregulated periplasmic glucans in Proteobacteria. FEMS Microbiology Letters, 186(1), 11–19. https://doi.org/10.1016/S0378-1097(00)00110-5
Oetiker, N. et al. (2018). Possible role of envelope components in the extreme copper resistance of the biomining Acidithiobacillus ferrooxidans. Genes (Basel), 9(7). https://doi.org/10.3390/genes9070347.
Rago, I., et al. (2014). Zinc oxide microrods and nanorods: Different antibacterial activity and their mode of action against Gram-positive bacteria. RSC Advances, 4(99), 56031–56040. https://doi.org/10.1039/c4ra08462d
Moussatova, A., Kandt, C., O’Mara, M. L., & Tieleman, D. P. (2008). ATP-binding cassette transporters in Escherichia coli. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1778(9), 1757–1771. https://doi.org/10.1016/j.bbamem.2008.06.009
Sklar, J. G., Wu, T., Kahne, D., & Silhavy, T. J. (2007). Defining the roles of the periplasmic chaperones SurA, Skp, and DegP in Escherichia coli. Genes & Development, 21(19), 2473–2484. https://doi.org/10.1101/gad.1581007
Lazar, S. W., & Kolter, R. (1996). SurA assists the folding of Escherichia coli outer membrane proteins. Journal of Bacteriology, 178(6), 1770–1773. https://doi.org/10.1128/jb.178.6.1770-1773.1996
Vertommen, D., Ruiz, N., Leverrier, P., Silhavy, T. J., & Collet, J. F. (2009). Characterization of the role of the escherichia coli periplasmic chaperone SurA using differential proteomics. Proteomics, 9(9), 2432–2443. https://doi.org/10.1002/pmic.200800794
Denoncin, K., Schwalm, J., Vertommen, D., & Silhavy, T. J. (2012). Dissecting the Escherichia coli periplasmic chaperone network using differential proteomics. Proteomics, 12(9), 1–21. https://doi.org/10.1002/pmic.201100633
Yakhnina, A. A., & Bernhardt, T. G. (2020). The Tol-Pal system is required for peptidoglycan-cleaving enzymes to complete bacterial cell division. Proceedings of the National Academy of Sciences, 117(12), 6777–6783. https://doi.org/10.1073/pnas.1919267117
Lloubès, R., et al. (2001). The Tol-Pal proteins of the Escherichia coli cell envelope: An energized system required for outer membrane integrity? Research in Microbiology, 152(6), 523–529. https://doi.org/10.1016/S0923-2508(01)01226-8
Szczepaniak, J., Press, C., & Kleanthous, C. (2020). The multifarious roles of Tol-Pal in Gram-negative bacteria. FEMS Microbiology Reviews, 44(4), 490–506. https://doi.org/10.1093/femsre/fuaa018
Carr, S., Penfold, C. N., Bamford, V., James, R., & Hemmings, A. M. (2000). The structure of TolB, an essential component of the tol-dependent translocation system, and its protein-protein interaction with the translocation domain of colicin E9. Structure, 8(1), 57–66. https://doi.org/10.1016/S0969-2126(00)00079-4
Kim, K. H., Aulakh, S., & Paetzel, M. (2012). The bacterial outer membrane β-barrel assembly machinery. Protein Science, 21(6), 751–768. https://doi.org/10.1002/pro.2069
Lee, J. H., & Lee, J. (2010). Indole as an intercellular signal in microbial communities. FEMS Microbiology Reviews, 34(4), 426–444. https://doi.org/10.1111/j.1574-6976.2009.00204.x
Basurko, Marie-Jose., Marche, Michele, Darriet, Monique, & Cassaigne, Andre. (1999). Phosphoserine aminotransferase, the second step–catalyzing enzyme for serine biosynthesis. IUBMB Life, 48, 525–529.
Gu, P., Yang, F., Su, T., Li, F., Li, Y., & Qi, Q. (2014). Construction of an L-serine producing Escherichia coli via metabolic engineering. Journal of Industrial Microbiology and Biotechnology, 41(9), 1443–1450. https://doi.org/10.1007/s10295-014-1476-6
Lüders, S., Fallet, C., & Franco-Lara, E. (2009). Proteome analysis of the Escherichia coli heat shock response under steady-state conditions. Proteome Science, 7, 36. https://doi.org/10.1186/1477-5956-7-36
Segura, A., et al. (2012). Solvent tolerance in Gram-negative bacteria. Current Opinion in Biotechnology, 23(3), 415–421. https://doi.org/10.1016/j.copbio.2011.11.015
Shen, J. S. Y., Rudolph, J., & Stern, M. (1990). Glycinamide ribonucleotide synthetase from Escherichia coli: Cloning, overproduction, sequencing, isolation, and characterization+. American Chemical Society Biochemistry, 29(1), 218–227.
Matuła, K., et al. (2019). Phenotypic plasticity of Escherichia coli upon exposure to physical stress induced by ZnO nanorods. Science and Reports, 9(1), 1–12. https://doi.org/10.1038/s41598-019-44727-w
Neal, A. L., Kabengi, N., Grider, A., & Bertsch, P. M. (2012). Can the soil bacterium Cupriavidus necator sense ZnO nanomaterials and aqueous Zn2+ differentially? Nanotoxicology, 6(4), 371–380. https://doi.org/10.3109/17435390.2011.579633
Sohm, B., Immel, F., Bauda, P., & Pagnout, C. (2015). Insight into the primary mode of action of TiO2 nanoparticles on Escherichia coli in the dark. Proteomics, 15(1), 98–113. https://doi.org/10.1002/pmic.201400101
Pathakoti, K., Manubolu, M., min Hwang, H. (2020). Mechanistic insights into TiO2 and ZnO nanoparticle-induced metabolic changes in Escherichia coli under solar simulated light irradiation. Water, Air, & Soil Pollution, 231(1). https://doi.org/10.1007/s11270-019-4388-2
Acknowledgements
The authors are thankful to Prof. Vivek Polshettiwar, Prof. Deepa Khushalani, and Mr. Balasaheb Chandanshive for helping in carrying out characterization experiments. The authors are also thankful to Mr. Bharat T Kansara for providing technical assistance in LCMS studies and for analyzing Proteomics data. The authors also acknowledge National Centre for Nanosciences and Nanotechnology, University of Mumbai (NCNNUM), for providing the nanoparticle characterization facilities.
Funding
This work was supported by the Tata Institute of Fundamental Research, Mumbai, and funded by the Department of Atomic Energy, Government of India, under project identification no. RT14003).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jaiswal, P.B., Jejurikar, S., Mondal, A. et al. Antibacterial Effects of ZnO Nanodisks: Shape Effect of the Nanostructure on the Lethality in Escherichia coli. Appl Biochem Biotechnol 195, 3067–3095 (2023). https://doi.org/10.1007/s12010-022-04265-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04265-0