Abstract
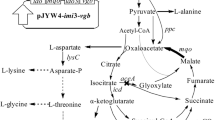
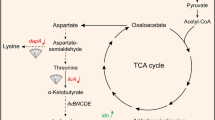
Corynebacterium glutamicum is the workhorse for the production of amino acids, including l-isoleucine (Ile). During Ile biosynthesis, NADPH is required as a crucial cofactor. In this study, four NADPH-supplying strategies based on NAD kinase, NADH kinase, glucose-6-phosphate dehydrogenase, and NAD kinase coupling with glucose-6-phosphate dehydrogenase were compared, and their influences on Ile biosynthesis were examined. PpnK is a NAD kinase of C. glutamicum ssp. lactofermentum JHI3-156 that predominantly phosphorylates NAD+ to produce NADP+. Pos5 is a NADH kinase of Saccharomyces cerevisiae that predominantly phosphorylates NADH to produce NADPH. Zwf is a glucose-6-phosphate dehydrogenase of JHI3-156. The ppnK, POS5, zwf, and zwf-ppnK genes were overexpressed in the Ile-producing strain JHI3-156. The expression of all four genes increased intracellular NADPH concentration and Ile production. The increase of NADPH concentration and Ile production in a POS5-expressing strain (229 and 75.6 %, respectively) was higher than that in a ppnK-expression strain. The expression of zwf also increased NADPH supply and Ile biosynthesis, but the constitutive expression of zwf was not as effective as the inducible expression of zwf. Coexpression of zwf and ppnK genes greatly enhanced NADPH supply and thus improved Ile production by up to 85.9 %, indicating that this strategy was the most effective one. These results are helpful for improving Ile biosynthesis and other biosynthetic processes.



Similar content being viewed by others
References
Hermann, T. (2003). Industrial production of amino acids by coryneform bacteria. Journal of Biotechnology, 104, 155–172.
Schneider, J., Niermann, K., & Wendisch, V. F. (2010). Production of the amino acids l-glutamate, l-lysine, l-ornithine and l-arginine from arabinose by recombinant Corynebacterium glutamicum. Journal of Biotechnology, 154, 191–198.
Park, J. H., & Lee, S. Y. (2010). Metabolic pathways and fermentative production of l-aspartate family amino acids. Biotechnology Journal, 5, 560–577.
Park, J. H., & Lee, S. Y. (2010). Fermentative production of branched chain amino acids: a focus on metabolic engineering. Applied Microbiology and Biotechnology, 85, 491–506.
Lordelo, M. M., Gaspar, A. M., Le, B. L., & Freire, J. P. (2008). Isoleucine and valine supplementation of a low-protein corn-wheat-soybean meal-based diet for piglets: growth performance and nitrogen balance. Journal of Animal Science, 86, 2936–2941.
Olde, D. S., Jalan, R., Deutz, N. E., Dejong, C. H., Redhead, D. N., Hynd, P., et al. (2007). Isoleucine infusion during "simulated" upper gastrointestinal bleeding improves liver and muscle protein synthesis in cirrhotic patients. Hepatology, 45, 560–568.
Guillouet, S., Rodal, A. A., An, G. H., Gorret, N., Lessard, P. A., & Sinskey, A. J. (2001). Metabolic redirection of carbon flow toward isoleucine by expressing a catabolic threonine dehydratase in a threonine-overproducing Corynabacterium glutamicum. Applied Microbiology and Biotechnology, 57, 667–673.
Yin, L. H., Hu, X. Q., Xu, D. Q., Ning, J. F., Chen, J., & Wang, X. Y. (2012). Co-expression of feedback-resistant threonine dehydratase and acetohydroxy acid synthase increase l-isoleucine production in Corynebacterium glutamicum. Metabolic Engineering, 14, 542–550.
Xie, X. X., Xu, L. L., Shi, J. M., Xu, Q. Y., & Chen, N. (2012). Effect of transport proteins on l-isoleucine production with the l-isoleucine-producing strain Corynebacterium glutamicum YILW. Journal of Industrial Microbiology and Biotechnology, 39, 1549–1556.
Moritz, B., Striegel, K., de Graaf, A. A., & Sahm, H. (2000). Kinetic properties of the glucose-6-phosphate and 6-phosphogluconate dehydrogenases from Corynebacterium glutamicum and their application for predicting pentose pathway flux in vivo. European Journal of Biochemistry, 267, 3442–3452.
Becker, J., Klopprogge, C., Herold, A., Zelder, O., Bolten, C. J., & Wittmann, C. (2007). Metabolic flux engineering of l-lysine production in Corynebacterium glutamicum—over expression and modification of G6P dehydrogenase. Journal of Biotechnology, 132, 99–109.
Georgi, T., Rittmann, D., & Wendisch, V. F. (2005). Lysine and glutamate production by Corynebacterium glutamicum on glucose, fructose and sucrose: roles of malic enzyme and fructose-1,6-bisphosphatase. Metabolic Engineering, 7, 291–301.
Bartek, T., Blombach, B., Lang, S., Eikmanns, B. J., Wiechert, W., Oldiges, M., et al. (2011). Comparative 13C metabolic flux analysis of pyruvate dehydrogenase complex-deficient, l-valine-producing Corynebacterium glutamicum. Applied and Environmental Microbiology, 77, 6644–6652.
Ying, W. (2008). NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxidants & Redox Signaling, 10, 179–206.
Garavaglia, S., Raffaelli, N., Finaurini, L., Magni, G., & Rizzi, M. (2004). A novel fold revealed by Mycobacterium tuberculosis NAD kinase, a key allosteric enzyme in NADP biosynthesis. Journal of Biological Chemistry, 279, 40980–40986.
Shi, F., Li, Y. F., Li, Y., & Wang, X. Y. (2009). Molecular properties, functions and potential applications of NAD kinases. Acta Biochimica et Biophysica Sinica, 41, 351–361.
Kawai, S., & Murata, K. (2008). Structure and function of NAD kinase and NADP phosphatase: key enzymes that regulate the intracellular balance of NAD(H) and NADP(H). Bioscience, Biotechnology, and Biochemistry, 72, 919–930.
Kawai, S., Mori, S., Mukai, T., Suzuki, S., Hashimoto, W., Tamada, T., & Murata, K. (2000). Inorganic polyphosphate/ATP-NAD kinase of Micrococcus flavus and Mycobacterium tuberculosis H37Rv. Biochemical and Biophysical Research Communications, 276, 57–63.
Lindner, S. N., Niederholtmeyer, H., Schmitz, K., Schoberth, S. M., & Wendisch, V. F. (2010). Polyphosphate/ATP-dependent NAD kinase of Corynebacterium glutamicum: biochemical properties and impact of ppnK overexpression on lysine production. Applied Microbiology and Biotechnology, 87, 583–593.
Ohashi, K., Kawai, S., & Murata, K. (2012). Identification and characterization of a human mitochondrial NAD kinase. Nature Communications, 3, 1248.
Shi, F., Huan, X. J., Wang, X. Y., & Ning, J. F. (2012). Overexpression of NAD kinases improves the l-isoleucine biosynthesis in Corynebacterium glutamicum ssp. lactofermentum. Enzyme and Microbial Technology, 51, 73–80.
Lee, H. C., Kim, J. S., Jang, W., & Kim, S. Y. (2009). Thymidine production by overexpressing NAD+ kinase in an Escherichia coli recombinant strain. Biotechnology Letters, 31, 1929–1936.
Outten, C. E., & Culotta, V. C. (2003). A novel NADH kinase is the mitochondrial source of NADPH in Saccharomyces cerevisiae. EMBO Journal, 22, 2015–2024.
Shi, F., Kawai, S., Mori, S., Kono, E., & Murata, K. (2005). Identification of ATP-NADH kinase isozymes and their contribution to supply of NADP(H) in Saccharomyces cerevisiae. FEBS Journal, 272, 3337–3349.
Miyagi, H., Kawai, S., & Murata, K. (2009). Two sources of mitochondrial NADPH in the yeast Saccharomyces cerevisiae. Journal of Biological Chemistry, 284, 7553–7560.
Shi, F., Li, Z. J., Sun, M. D., & Li, Y. F. (2011). The role of mitochondrial NADH kinase and NADPH supply in respiratory chain activity of Saccharomyces cerevisiae. Acta Biochimica et Biophysica Sinica, 43, 989–995.
Strand, M. K., Stuart, G. R., Longley, M. J., Graziewicz, M. A., Dominick, O. C., & Copeland, W. C. (2003). POS5 gene of Saccharomyces cerevisiae encodes a mitochondrial NADH kinase required for stability of mitochondrial DNA. Eukaryotic Cell, 2, 809–820.
Xu, D. Q., Tan, Y. Z., Huan, X. J., Hu, X. Q., & Wang, X. Y. (2010). Construction of a novel shuttle vector for use in Brevibacterium flavum, an industrial amino acid producer. Journal of Microbiological Methods, 80, 86–92.
Xu, D. Q., Tan, Y. Z., Shi, F., & Wang, X. Y. (2010). An improved shuttle vector constructed for metabolic engineering research in Corynebacterium glutamicum. Plasmid, 64, 85–91.
Blombach, B., Schreiner, M. E., Holatko, J., Bartek, T., Oldiges, M., & Eikmanns, B. J. (2007). l-valine production with pyruvate dehydrogenase complex-deficient Corynebacterium glutamicum. Applied and Environment Microbiology, 73, 2079–2084.
Lee, I. Y., Kim, M. K., Park, Y. H., & Lee, S. Y. (1996). Regulatory effects of cellular nicotinamide nucleotides and enzyme activities on poly(3-hydroxybutyrate) synthesis in recombinant Escherichia coli. Biotechnology and Bioengineering, 52, 707–712.
Kennerknecht, N., Sahm, H., Yen, M. R., Pátek, M., Saier, M. H., Jr., & Eggeling, L. (2002). Export of l-isoleucine from Corynebacterium glutamicum: a two-gene-encoded member of a new translocator family. Journal of Bacteriology, 184, 3947–3956.
Bartek, T., Blombach, B., Zonnchen, E., Makus, P., Lang, S., Eikmanns, B. J., et al. (2010). Importance of NADPH supply for improved l-valine formation in Corynebacterium glutamicum. Biotechnology Progress, 26, 361–371.
Lee, W. H., Kim, J. W., Park, E. H., Han, N. S., Kim, M. D., & Seo, J. H. (2013). Effects of NADH kinase on NADPH-dependent biotransformation processes in Escherichia coli. Applied Microbiology and Biotechnology, 97, 1561–1569.
Shi, A., Zhu, X., Lu, J., Zhang, X., & Ma, Y. (2013). Activating transhydrogenase and NAD kinase in combination for improving isobutanol production. Metabolic Engineering, 16, 1–10.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (no. 30870056).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, F., Li, K., Huan, X. et al. Expression of NAD(H) Kinase and Glucose-6-Phosphate Dehydrogenase Improve NADPH Supply and l-isoleucine Biosynthesis in Corynebacterium glutamicum ssp. lactofermentum . Appl Biochem Biotechnol 171, 504–521 (2013). https://doi.org/10.1007/s12010-013-0389-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0389-6