Abstract
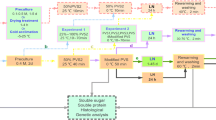
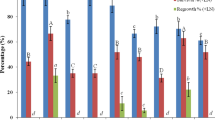
Regrowth of the cryopreserved protocorm-like bodies (PLBs) of Dendrobium Bobby Messina was assessed based on the plant vitrification solution 2 (PVS2) optimisation conditions. The optimized protocol obtained based on TTC spectrophotometrical analysis and growth recovery were 3–4 mm of PLBs size precultured in 0.2 M sucrose for 1 day, treated with a mixture of 2 M glycerol and 0.4 M sucrose supplemented with half-strength liquid MS media at 25 °C for 20 min and subsequently dehydrated with PVS2 at 0 °C for 20 min prior to storage in liquid nitrogen. Following rapid warming in a water bath at 40 °C for 90 s, PLBs were treated with unloading solution containing half-strength liquid MS media supplemented with 1.2 M sucrose. Subsequently, the PLBs were cultured on half-strength semi-solid MS media supplemented with 2 % (w/v) sucrose without any growth regulators and resulted in 40 % growth recovery. In addition, ascorbic acid treatment was used to evaluate the regeneration process of cryopreserved PLBs. However, growth recovery rates of non-cryopreserved and cryopreserved PLBs were 30 and 10 % when 0.6 mM ascorbic acid was added. Scanning electron microscopy analysis indicates that there are not much damages observed on both cryopreserved and non-cryopreserved PLBs in comparison to PLBs stock culture.







Similar content being viewed by others
Abbreviations
- PLB:
-
Protocorm-like bodies
- PVS2:
-
Plant vitrification solution
- + LN:
-
Cryopreserved PLBs
- − LN:
-
Non-cryopreserved PLB
References
Godo, T., Komori, M., Nakaoki, E., Yukawa, T., & Miyoshi, K. (2010). In Vitro Cellular & Developmental Biology. Plant, 46, 323–328.
Bajaj, Y. P. S. (1995). In Y. P. S. Bajaj (Ed.), Biotechnology in agriculture and forestry cryopreservation of plant germplasm, vol 1: Cryopreservation of plant cell, tissue and organ culture for the conservation of germplasm and biodiversity (pp. 3–18). New York: Springer.
Towill, L. E. (1996). In R. S. Trigiano & D. J. Gray (Eds.), Plant tissue culture concepts and laboratory exercises, vitrification as a method to cryopreserve shoot tips (pp. 297–304). Boca Raton: CRC Press.
Engelmann, F. (2000). in Cryopreservation of tropical germplasm Current Research Progress and Application, Importance of cryopreservation for the conservation of plant genetic resources (Engelmann, F. & Takagi, H., eds), Japan International Research Center for Agricultural Sciences and International Plant Genetic Resources Institute, Rome, pp. 8–20.
Burritt, D. J. (2008). Plant Cell, Tissue and Organ Culture, 95, 209–215.
Engelmann, F. (1997). Plant Genetic Resources Newsletter, 112, 9–18.
Martinez, D., Tames, S. R., & Revilla, A. M. (1999). Plant Cell Report, 19, 59–63.
Sakai, A., Matsumoto, T., Hirai, D., & Niino, T. (2000). Cryo-Letters, 21, 53–62.
Gonzalez-Arnao, M. T., Panta, A., Roca, W. M., Escobar, R. H., & Engelmann, F. (2008). Plant Cell, Tissue and Organ Culture, 92, 1–13.
Khoddamzadeh, A. A., Sinniah, U. R., Lynch, P., Kadir, M. A., Kadzimin, S. B., & Mahmood, M. (2011). Plant Cell, Tissue and Organ Culture, 107, 471–481.
Hirano, T., Ishikawa, K., & Mii, M. (2006). In J. A. T. da Silva (Ed.), Floriculture, ornamental and plant biotechnology, advances and topical issues, vol. II: Advances in orchid cryopreservation (pp. 410–414). Ikenobe: Global Science Books.
Tsai, S. F., Yeh, S. D., Chan, C. F., & Liaw, S. I. (2009). Plant Cell, Tissue and Organ Culture, 98, 157–164.
Wusteman, M., Pegg, D., Robinson, M., Wang, L., & Fitch, P. (2002). Cryobiology, 44, 24–37.
Na, Y. H., & Kondo, K. (1996). Plant Science, 118, 195–201.
Ishikawa, K., Harata, K., Mii, M., Sakai, A., Yoshimatsu, K., & Shimomura, K. (1997). Plant Cell Reports, 16, 754–757.
Hirano, T., Ishikawa, K., & Mii, M. (2005). Cryo-Letters, 26, 139–146.
Datta, B. K., Kanjilal, B., & Sarker, D. D. (1999). Current Science, 74, 1142–1145.
Luo, J. P., Wang, Y., Zha, X. Q., & Huang, L. (2008). Plant Cell, Tissue and Organ Culture, 93, 333–340.
Tsukazaki, H., Mii, M., Tokuhara, K., & Ishikawa, K. (2000). Plant Cell Reports, 19, 1160–1164.
Lurswijidjarus, W., & Thammasiri, K. (2004). Science Asia, 30, 293–299.
Thammasiri, K. (2000). Cryo-Letters, 21, 237–244.
Hirano, T., Godo, T., Mii, M., & Ishikawa, K. (2005). Plant Cell Reports, 23, 534–539.
Gonzalez-Arnao, M. T., Lazara-Vallejo, C. E., Engelmann, F., Gamez-Pastrana, R., Martinez-Ocampo, Y. M., Pastelin-Solano, M. C., et al. (2009). In Vitro Cellular & Developmental Biology. Plant, 45, 574–582.
Murashige, T., & Skoog, F. (1962). Physiologia Plantarum, 15, 473–497.
Sakai, A., Kobayashi, S., & Oiyama, I. (1990). Plant Cell Reports, 9, 30–33.
Sakai, A., Koboyashi, S., & Oiyama, I. (1991). Journal of Plant Physiology, 137, 465–470.
Verleysen, H., Samyn, G., Bockstaele, E. V., & Debergh, P. (2004). Plant Cell, Tissue and Organ Culture, 77, 11–21.
Benson, E. E., Johnson, J., Muthusamy, J., & Harding, K. (2008). In S. D. Gupta & Y. Ibaraki (Eds.), Physical and engineering perspectives of in vitro plant cryopreservation, plant tissue culture engineering (pp. 441–476). Malaysia: Springer.
Day, J. G., Harding, K. C., Nadarajan, J., & Benson, E. E. (2008). Cryopreservation: Conservation of bioresources at ultra low temperatures. In J. M. Walker & R. Rapley (Eds.), Molecular biomethods handbook (pp. 917–947). Totowa: Humana Press.
Panis, B., & Lambardi, M. (2005). International workshop on “The role of biotechnology for characterization and conservation of crop, forestry, animal and fishery genetic resources”, Turin, Italy, pp. 43–54.
Panis, B. (2008). In B. M. Reed (Ed.), Plant cryopreservation, a practical guide, Cryopreservation of monocots (pp. 241–251). New York: Springer.
Walters, C., Farrant, J. M., Pammenter, N. W., & Berjak, P. (2002). In M. Black & H. W. Pritchard (Eds.), Desiccation and survival in plants: drying without dying, desiccation stress and damage (pp. 263–291). Oxford: Oxford University Press.
Subramaniam, S., Sinniah, U. R., Khoddamzadeh, A. A., Periasamy, S., & James, J. J. (2011). African Journal of Biotechnology, 10, 3902–3907.
Yin, L. L., Poobathy, R., James, J., Julkifle, A. L., & Subramaniam, S. (2011). African Journal of Biotechnology, 10, 4665–4672.
Wang, Q., Tanne, E., Arav, A., & Gafny, R. (2000). Plant Cell, Tissue and Organ Culture, 63, 41–46.
Antony, J. J. J., Lai Keng, C., Rathinam, X., & Subramaniam, S. (2010). African Journal of Biotechnology, 9, 7063–7070.
Antony, J. J. J., Keng, C. L., Rathinam, X., Marimuthu, S., & Subramaniam, S. (2011). Australian Journal of Crop Science, 5, 1557–1564.
Thinh, N. T. (1997). PhD Thesis, Faculty of Agriculture, Kobe University, Japan.
Nishizawa, S., Sakai, A., Amano, Y., & Matsuzawa, T. (1993). Plant Science, 91, 67–73.
Kuranuki, Y., & Sakai, A. (1995). CryoLetters, 16, 345–352.
Yoshimatsu, K., Yamaguchi, H., & Shimomura, K. (1996). Plant Cell Reports, 15, 555–560.
Matsumoto, T., Sakai, A., & Yamada, K. (1994). Plant Cell Reports, 13, 442–446.
Takagi, H., Thinh, N. T., Sakai, A., & Senboku, T. (1997). Plant Cell Reports, 16, 594–599.
Niino, T., Sakai, A., Yakuwa, H., & Nojiri, K. (1992). Plant Cell, Tissue and Organ Culture, 28, 261–266.
Niino, T., Takano, J., Saga, T., & Kobayashi, M. (2003). Horticultural Research (Japan), 2, 241–245.
Dussert, S., Engelmann, F., & Noirot, M. (2003). CryoLetters, 24, 149–160.
Uchendu, E. E., Leonard, S. W., Traber, M. G., & Reed, B. M. (2009). Plant Cell Reports, 29, 25–35.
Halliwell, B., & Gutteridge, J. M. C. (1984). Biochemical Journal, 219, 1–14.
Halliwell, B. (2006). Plant Physiology, 141, 312–322.
Zheng, Q., Ju, B., Liang, L., & Xiao, X. (2005). Plant Cell, Tissue and Organ Culture, 81, 83–90.
Thammasiri, K., & Soamkul, L. (2007). Science Asia, 33, 223–227.
Acknowledgments
This work was supported by Universiti Sains Malaysia Research Grant 2011 (USM) and National Science Fellowship (NSF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Antony, J.J.J., Keng, C.L., Mahmood, M. et al. Effects of Ascorbic Acid on PVS2 Cryopreservation of Dendrobium Bobby Messina’s PLBs Supported with SEM Analysis. Appl Biochem Biotechnol 171, 315–329 (2013). https://doi.org/10.1007/s12010-013-0369-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0369-x