Abstract
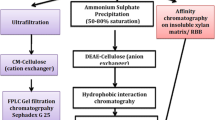
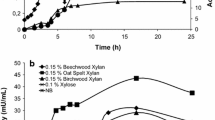
A highly thermostable alkaline xylanase was purified to homogeneity from culture supernatant of Bacillus sp. JB 99 using DEAE-Sepharose and Sephadex G-100 gel filtration with 25.7-fold increase in activity and 43.5% recovery. The molecular weight of the purified xylanase was found to be 20 kDA by SDS-PAGE and zymogram analysis. The enzyme was optimally active at 70 °C, pH 8.0 and stable over pH range of 6.0–10.0.The relative activity at 9.0 and 10.0 were 90% and 85% of that of pH 8.0, respectively. The enzyme showed high thermal stability at 60 °C with 95% of its activity after 5 h. The K m and V max of enzyme for oat spelt xylan were 4.8 mg/ml and 218.6 µM min−1 mg−1, respectively. Analysis of N-terminal amino acid sequence revealed that the xylanase belongs to glycosyl hydrolase family 11 from thermoalkalophilic Bacillus sp. with basic pI. Substrate specificity showed a high activity on xylan-containing substrate and cellulase-free nature. The hydrolyzed product pattern of oat spelt xylan on thin-layer chromatography suggested xylanase as an endoxylanase. Due to these properties, xylanase from Bacillus sp. JB 99 was found to be highly compatible for paper and pulp industry.





Similar content being viewed by others
References
Beg, Q. K., Kapoor, M., Mahajan, L., & Hoondal, G. S. (2001). Applied Microbiology and Biotechnology, 56, 326–338. doi:10.1007/s002530100704.
Gessesse, A., & Mamo, G. (1999). Enzyme and Microbial Technology, 25, 68–72. doi:10.1016/S0141-0229(99)00006-X.
Takahashi, H., Nakai, R., & Nakamura, S. (2000). Biosci Biotechnol Biochem, 64, 887–890.
Johnvesly, B., & Naik, G. R. (2001). Process Biochemistry, 37, 139–144. doi:10.1016/S0032-9592(01)00191-1.
Johnvesly, B., Virupakshi, S., Patil, G. N., Ramalingam, & Naik, G. R. (2002). J Microbiol Biotechnol, 12, 53–156.
Ratanakhanokchai, K., Kyu, K. L., & Tanticharoen, M. (1999). Appl Environ Microbiol, 65, 694–697.
Laemmli, U. K. (1970). Nature, 227, 680–686. doi:10.1038/227680a0.
Maalej, I., Belhaj, I., Masmoudi, N. F., & Belghith, H. (2009). Applied Biochemistry and Biotechnology, 158, 200–212. doi:10.1007/s12010-008-8317-x.
Monica, D., Castro, A., Castro, R. M., Andrade, C. M., & Pereira, N. (2004). Applied Biochemistry and Biotechnology, 115, 1–3. doi:10.1385/ABAB:115:1-3:1003.
Chang, P., Tsai, W.-S., Tsai, C.-L., & Tseng, M.-J. (2004). Biochem Biophys Res Comms, 319, 1017–1025. doi:10.1016/j.bbrc.2004.05.078.
Sudan, R., & Bajaj, B. K. (2007). World J Microbiol Biotechnol, 23, 491–500. doi:10.1007/s11274-006-9251-0.
Nakamura, S., Wakabayashi, K., & Horikoshi, K. (1993). Appl Environ Microbiol, 59, 2311–2316.
Sharma, A., Adhikari, S., & Satyanarayana, T. (2007). World Journal of Microbiology & Biotechnology, 23, 483–490. doi:10.1007/s11274-006-9250-1.
Subramaniyan, S., & Prema, P. (2000). FEMS Microbiology Letters, 183, 1–7. doi:10.1111/j.1574-6968.2000.tb08925.x.
Subramaniyan, S., & Prema, P. (2002). Critical Reviews in Biotechnology, 22, 33–64. doi:10.1080/07388550290789450.
Collins, T., Gerday, C., & Feller, G. (2005). FEMS Microbiology, 29, 3–23. doi:10.1016/j.femsre.2004.06.005.
Viikari, L., Kantelinen, A., Buchert, J., & Puls, J. (1994). Applied Microbiology and Biotechnology, 41, 124–129. doi:10.1007/BF00166093.
Virupakshi, S., Girresh, Babu, Satish, G. R., & Naik, G. R. (2005). Process Biochemistry, 40, 431–435. doi:10.1016/j.procbio.2004.01.027.
Acknowledgments
The authors would like to thank the Council of Scientific and Industrial Research, New Delhi (Scheme no. 37/1297/07 EMR-II) for the financial support and providing Mr. D. Shrinivas the Junior Research Fellowship. The authors would also like to thank the National Facility for Protein Sequencing (Indian Institute of Technology, Mumbai) in carrying out protein sequencing work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shrinivas, D., Savitha, G., Raviranjan, K. et al. A Highly Thermostable Alkaline Cellulase-Free Xylanase from Thermoalkalophilic Bacillus sp. JB 99 Suitable for Paper and Pulp Industry: Purification and Characterization. Appl Biochem Biotechnol 162, 2049–2057 (2010). https://doi.org/10.1007/s12010-010-8980-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-010-8980-6