Abstract
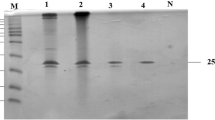
A thermostable xylanase from a newly isolated thermophilic fungus Talaromyces thermophilus was purified and characterized. The enzyme was purified to homogeneity by ammonium sulfate precipitation, diethylaminoethyl cellulose anion exchange chromatography, P-100 gel filtration, and Mono Q chromatography with a 23-fold increase in specific activity and 17.5% recovery. The molecular weight of the xylanase was estimated to be 25kDa by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and gel filtration. The enzyme was highly active over a wide range of pH from 4.0 to 10.0. The relative activities at pH5.0, 9.0, and 10.0 were about 80%, 85.0%, and 60% of that at pH7.5, respectively. The optimum temperature of the purified enzyme was 75°C. The enzyme showed high thermal stability at 50°C (7days) and the half-life of the xylanase at 100°C was 60min. The enzyme was free from cellulase activity. K m and V max values at 50°C of the purified enzyme for birchwood xylan were 22.51mg/ml and 1.235μmol min−1 mg−1, respectively. The enzyme was activated by Ag+, Co2+, and Cu2+; on the other hand, Hg2+, Ba2+, and Mn2+ inhibited the enzyme. The present study is among the first works to examine and describe a secreted, cellulase-free, and highly thermostable xylanase from the T. thermophilus fungus whose application as a pre-bleaching aid is of apparent importance for pulp and paper industries.









Similar content being viewed by others
References
Bastawde, K. B. (1992). World Journal of Microbiology & Biotechnology, 8, 353–368. doi:10.1007/BF01198746.
Joseleau, J. P., Comtat, J., & Rue, l. K. (1992). Progress in Biotechnology, 7, 1–15.
Biely, P. (1985). Trends in Biotechnology, 3, 286–290. doi:10.1016/0167-7799(85)90004-6.
Kulkarni, N., Shendye, A., & Rao, M. (1999). FEMS Microbiology Reviews, 23, 411–456. doi:10.1111/j.1574-6976.1999.tb00407.x.
Beg, Q. K., Kapoor, M., Mahajan, L., & Hoondal, G. S. (2001). Applied Microbiology and Biotechnology, 56, 326–338. doi:10.1007/s002530100704.
Zaldivar, J., Nielsen, J., & Olsson, L. (2001). Applied Microbiology and Biotechnology, 56, 17–34. doi:10.1007/s002530100624.
Yang, X., Chen, H., Gao, H., & Li, Z. (2001). Bioresource Technology, 78, 277–280. doi:10.1016/S0960-8524(01)00024-4.
Maat, J., Roza, M., Verbakel, J., Stam, H., Santos de Silva, M. J., & Bosse, M. (1992). Amsterdam, The Netherlands: Elsevier, pp. 349–360.
Madlala, A. M., Bissoon, S., Singh, S., & Christov, L. (2001). Biotechnology Letters, 23, 345–351. doi:10.1023/A:1005693205016.
Tuohy, M. G., & Coughlan, M. P. (1992). Bioresource Technology, 39, 131–137. doi:10.1016/0960-8524(92)90131-G.
Haltrich, D., Nidetzky, B., Kulbe, K. D., Steiner, W., & Zupancic, S. (1996). Bioresource Technology, 58, 137–161.
Kuhad, R. C., & Singh, A. (1993). Critical Reviews in Biotechnology, 13, 151–172. doi:10.3109/07388559309040630.
Yu, E. K. C., Tan, L. U. L., Chan, M. K., & Saddler, J. N. (1993). Enzyme and Microbial Technology, 9, 16–24. doi:10.1016/0141-0229(87)90044-5.
Coughlan, M. P., Tuohy, M. G., Filho, E. X. F., Puls, J., Clayessens, M., Vrsanská, M., et al. (1993). In M. P. Coughlan & G. P.Hazlewood (Eds.) (pp. 53–84). London: Portland.
Gomes, J., Purkarthofer, H., Hany, M., Kapplmüller, J., Sinner, M., & Steiner, W. (1993). Applied Microbiology and Biotechnology, 39, 700–707. doi:10.1007/BF00164453.
Maheshwari, R., Bharadwaj, G., & Bhat, M. K. (2000). Microbiology and Molecular Biology Reviews, 64, 461–488. doi:10.1128/MMBR.64.3.461-488.2000.
Singh, S., Madlala, A. M., & Prior, B. A. (2003). FEMS Microbiology Reviews, 27, 3–16. doi:10.1016/S0168-6445(03)00018-4.
Lite, Li., Hongmei, T., Yongqiang, C., Zhengqiangand, J., & Shaoqing, Y. (2005). Enzyme and Microbial Technology, 38, 780–787.
Kelly, C. T., O’Mahony, M. R., & Fogarty, W. M. (1989). Biotechnology Letters, 11, 885–890. doi:10.1007/BF01026846.
Singh, S., Pillay, B., & Prior, B. A. T. (2000). Enzyme and Microbial Technology, 20, 502–508.
Mandels, M., & Weber, J. (1969). Advances in Chemistry Series, 95, 391–413.
Miller, G. L. (1959). Analytical Chemistry, 31, 426–428. doi::10.1021/ac60147a030.
Lacke, A. H. (1988). Methods in Enzymology, 160, 679–684.
Bradford, M. (1976). Analytical Biochemistry, 72, 248–254. doi:10.1016/0003-2697(76)90527-3.
Laemmli, U. K. (1970). Nature, 227, 680–685. doi:10.1038/227680a0.
Lineweaver, H., & Burk, D. (1934). Journal of the American Chemical Society, 56, 658–666. doi:10.1021/ja01318a036.
Wong, K. K. Y., Tan, L. U. L., & Saddler, J. N. (1988). Microbiological Reviews, 52, 305–317.
Yu, E. K. C., Tan, L. U. L., Chan, M. K.-H., Deschatelets, L., & John Saddler, N. (1987). Enzyme and Microbial Technology, 9, 16–24. doi:10.1016/0141-0229(87)90044-5.
Damaso Monica, C. T., Andrade, C. M. M. C., Nei, P. E. R. E. I., & Ra, J. R. (2002). Brazilian Journal of Microbiology, 33, 333–338. doi:10.1590/S1517-83822002000400011.
Cesar, T., & Mrsa, V. (1996). Enzyme and Microbial Technology, 19, 289–296. doi:10.1016/0141-0229(95)00248-0.
Acknowledgments
This work has been supported by grants from the Tunisian government’s “Contrat-Programme” through the “Ministère de la Recherche Scientifique, de la Technologie et du Développement des Compétences” of Tunisia. The authors wish to express their sincere gratitude to Prof. Ali Gargouri (Head of the Laboratory of Molecular Genetics of Eukaryotes, CBS Sfax Tunisia) for his critical reading and valuable suggestions during the experimental work. They also wish to extend their sincere thanks to Mr. Anouar Smaoui from FSS for his constructive editing of the current paper and careful proofreading of its Englishness.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maalej, I., Belhaj, I., Masmoudi, N.F. et al. Highly Thermostable Xylanase of the Thermophilic Fungus Talaromyces thermophilus: Purification and Characterization. Appl Biochem Biotechnol 158, 200–212 (2009). https://doi.org/10.1007/s12010-008-8317-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8317-x