Abstract
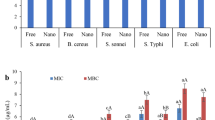
For the first time, a comparative study on antioxidant and antimicrobial properties of two lipid-based nanovesicles encapsulated myrtle extract, including different formulations of nanoliposome and nanoniosome, is conducted. GC–MS analysis of the hydroethanolic extract of myrtle leaf revealed 1,2,3-benzenetriol (40.62%) as a major component. It is worth mentioning that both nanovesicles were prepared in the absence of toxic solvents and cholesterol. The results of this study have displayed that as-fabricated nanovesicles were temperature and pH-sensitive. The differential scanning calorimetry (DSC) study showed a significant improvement in the thermal phase transition of the myrtle extract after encapsulation. In addition, the nanoniosome formulation was more stable than the nanoliposome formulation. The myrtle extract release rate was controlled in vitro by adjusting the wt. % of constituents in lipid and aqueous phases of the nanovesicles. The results have confirmed the fact that the release rate of extract from nanovesicles plays a crucial role in controlling biological activities, as was expected. The myrtle extract-encapsulated nanoliposomes with higher release rates exhibited more robust antioxidant activity compared to that of nanoniosomes. In the current work, antioxidant activities by FRAP were negligible compared with the DPPH method. The nanoliposome formulations showed the lowest MIC and highest zone inhibition against S. aureus, E.coli, and S. enteritidis compared to that of nanoniosome formulations.









Similar content being viewed by others
Data Availability
The authors declare that [the/all other] data supporting the findings of this study are available within the article.
References
Abdulkarim, S. M., & Ghazali, H. M. (2007). Comparison of melting behaviors of edible oils using conventional and hyper differential scanning calorimetric scan rates. ASEAN Food Journal, 14(1), 25.
Azarpazhooh, E., Sharayei, P., Zomorodi, S., & Ramaswamy, H. S. (2019). Physicochemical and phytochemical characterization and storage stability of freeze-dried encapsulated pomegranate peel anthocyanin and in vitro evaluation of its antioxidant activity. Food and Bioprocess Technology, 12(2), 199–210.
Aleksic, V., & Knezevic, P. (2014). Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiological Research, 169(4), 240–254.
Al-Laith, A. A., Alkhuzai, J., & Freije, A. (2019). Assessment of antioxidant activities of three wild medicinal plants from Bahrain. Arabian Journal of Chemistry, 12(8), 2365–2371.
Akyuz, M., Guzel, A., & Elmastas, M. (2019). Fatty acid composition and antioxidant capacity of Myrtus (Myrtus communis L.). Malaysian Applied Biology Journal, 48(5), 101–112.
Aziz, S. G. G., & Almasi, H. (2018). Physical characteristics, release properties, and antioxidant and antimicrobial activities of whey protein isolate films incorporated with thyme (Thymus vulgaris L.) extract-loaded nanoliposomes. Food and Bioprocess Technology, 11(8), 1552–1565.
Bartelds, R., Nematollahi, M. H., Pols, T., Stuart, M. C. A., Pardakhty, A., Asadikaram, G., Poolman, B. (2018) Niosomes, an alternative for liposomal delivery. PLOS ONE, 13(4), e0194179.
Basiri, L., Rajabzadeh, G., & Bostan, A. (2017). α-Tocopherol-loaded niosome prepared by heating method and its release behavior. Food Chemistry, 221, 620–628.
Brandelli, A. (2020). The interaction of nanostructured antimicrobials with biological systems: Cellular uptake, trafficking and potential toxicity. Food Science and Human Wellness, 9(1), 8–20.
Caddeo, C., Manconi, M., Fadda, A. M., Lai, F., Lampis, S., Diez-Sales, O., & Sinico, C. (2013). Nanocarriers for antioxidant resveratrol: Formulation approach, vesicle self-assembly, and stability evaluation. Colloids and Surfaces B: Bio Interfaces, 111, 327–332.
Chaves, N., Santiago, A., & Alías, J. C. (2020). Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants, 9(1), 76.
Dairi, S., Carbonneau, M. A., Galeano-Diaz, T., Remini, H., Dahmoune, F., Aoun, O., & Madani, K. (2017). Antioxidant effects of extra virgin olive oil-enriched by myrtle phenolic extracts on iron-mediated lipid peroxidation under intestinal conditions model. Food Chemistry, 237, 297–304.
Dave, V., Yadav, R. B., Kushwaha, K., Yadav, S., Sharma, S., & Agrawal, U. (2017). Lipid-polymer hybrid nanoparticles: Development & statistical optimization of norfloxacin for topical drug delivery system. Bioactive Materials, 2(4), 269–280.
de Moura, S. C., Schettini, G. N., Garcia, A. O., Gallina, D. A., Alvim, I. D., & Hubinger, M. D. (2019). Stability of hibiscus extract encapsulated by ionic gelation incorporated in yogurt. Food and Bioprocess Technology, 12(9), 1500–1515.
Erami, S. R., Amiri, Z. R., & Jafari, S. M. (2019). Nanoliposomal encapsulation of Bitter Gourd (Momordica charantia) fruit extract as a rich source of health-promoting bioactive compounds. LWT, 116, 108581.
Fathi, M., Varshosaz, J., Mohebbi, M., & Shahidi, F. (2013). Hesperetin-loaded solid lipid nanoparticles and nanostructure lipid carriers for food fortification: Preparation, characterization, and modeling. Food and Bioprocess Technology, 6(6), 1464–1475.
Gorjian, H., Amiri, Z. R., Milani, J. M., & Khaligh, N. G. (2021). Preparation and characterization of the encapsulated myrtle extract nanoliposome and nanoniosome without using cholesterol and toxic organic solvents: A Comparative Study. Food Chemistry, 128342.
Gortzi, O., Lalas, S., Chinou, I., & Tsaknis, J. (2008). Reevaluation of bioactivity and antioxidant activity of Myrtus communis extract before and after encapsulation in liposomes. European Food Research and Technology, 226(3), 583–590.
Hennia, A., Miguel, M. G., & Nemmiche, S. (2018). Antioxidant activity of Myrtus communis L. and Myrtus nivellei Batt. & Trab. extracts: A brief review. Medicines, 5(3), 89.
Huang, J., Wu, M., Yang, K., Zhao, M., Wu, D., Ma, J., & Sun, W. (2020). Effect of nanoliposomal entrapment on antioxidative hydrolysates from goose blood protein. Journal of Food Science, 85(10), 3034–3042.
Janakirama, A. R. R. S., Shivayogi, S. M., Satyanarayana, J. K., & Kumaran, R. C. (2021). Characterization of isolated compounds from Morus spp. and their biological activity as anticancer molecules. BioImpacts: BI, 11(3), 187.
Jansen-Alves, C., Krumreich, F. D., Zandoná, G. P., Gularte, M. A., Borges, C. D., & Zambiazi, R. C. (2019). Production of propolis extract microparticles with concentrated pea protein for application in food. Food and Bioprocess Technology, 12(5), 729–740.
Khameneh, B., Iranshahy, M., Soheili, V., & Bazzaz, B. S. F. (2019). Review on plant antimicrobials: A mechanistic viewpoint. Antimicrobial Resistance & Infection Control, 8(1), 1–28.
Khoee, S., & Yaghoobian, M. (2017). Niosomes: A novel approach in modern drug delivery systems. In Nanostructures for drug delivery. Elsevier. 207–237.
Kranenburg, M., & Smit, B. (2005). Phase behavior of model lipid bilayers. The Journal of Physical Chemistry B, 109(14), 6553–6563.
Lasic, D. D. (1993). Liposomes: From physics to application Elsevier. Amsterdam, New York.
Lewoyehu, M., & Amare, M. (2019). Comparative evaluation of analytical methods for determining the antioxidant activities of honey: A review. Cogent Food & Agriculture, 5(1), 1685059.
Mozafari, M. R., Johnson, C., Hatziantoniou, S., & Demetzos, C. (2008). Nanoliposomes and their applications in food nanotechnology. Journal of Liposome Research, 18(4), 309–327.
Ngamakeue, N., & Chitprasert, P. (2016). Encapsulation of holy basil essential oil in gelatin: Effects of palmitic acid in carboxymethyl cellulose emulsion coating on antioxidant and antimicrobial activities. Food and Bioprocess Technology, 9(10), 1735–1745.
Oliva, R., Chino, M., Pane, K., Pistorio, V., De Santis, A., Pizzo, E., & Petraccone, L. (2018). Exploring the role of unnatural amino acids in antimicrobial peptides. Scientific Reports, 8(1), 1–16.
Parvekar, P., Palaskar, J., Metgud, S., Maria, R., & Dutta, S. (2020). The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomaterial Investigations in Dentistry, 7(1), 105–109.
Pereira, J. A., Oliveira, I., Sousa, A., Valentão, P., Andrade, P. B., Ferreira, I. C., & Estevinho, L. (2007). Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity, and antioxidant potential of different cultivars. Food and Chemical Toxicology, 45(11), 2287–2295.
Pinilla, C. M. B., Reque, P. M., & Brandelli, A. (2020). Effect of oleic acid, cholesterol, and octadecylamine on membrane stability of freeze-dried liposomes encapsulating natural antimicrobials. Food and Bioprocess Technology, 13(4), 599–610.
Raeiszadeha, M., Pardakhtyc, A., Sharififara, F., Mehrabanid, M., Nejatmehrab-kermanie, H., & Mehrabani, M. (2018). Phytoniosome: A novel drug delivery for Myrtle extract. Iranian Journal of Pharmaceutical Research: IJPR, 17(3), 804.
Ribeiro, A. M., Estevinho, B. N., & Rocha, F. (2019). Spray drying encapsulation of elderberry extract and evaluating the release and stability of phenolic compounds in encapsulated powders. Food and Bioprocess Technology, 12(8), 1381–1394.
Rivero-Cruz, J. F., Granados-Pineda, J., Pedraza-Chaverri, J., Pérez-Rojas, J. M., Kumar-Passari, A., Diaz-Ruiz, G., & Rivero-Cruz, B. E. (2020). Phytochemical constituents, antioxidant, cytotoxic, and antimicrobial activities of the ethanolic extract of Mexican brown propolis. Antioxidants, 9(1), 70.
Rumbaoa, R. G. O., Cornago, D. F., & Geronimo, I. M. (2009). Phenolic content and antioxidant capacity of Philippine potato (Solanum tuberosum) tubers. Journal of Food Composition and Analysis, 22(6), 546–550.
Rutherford, D., Jíra, J., Kolářová, K., Matolínová, I., Mičová, J., Remeš, Z., & Rezek, B. (2021). Growth inhibition of gram-positive and gram-negative bacteria by zinc oxide hedgehog particles. International Journal of Nanomedicine, 16, 3541.
Savaghebi, D., Barzegar, M., & Mozafari, M. R. (2020). Manufacturing of nanoliposomal extract from Sargassum boveanum algae and investigating its release behavior and antioxidant activity. Food Science & Nutrition, 8(1), 299–310.
Saadat, S., Emam-Djomeh, Z., & Askari, G. (2021). Antibacterial and antioxidant gelatin nanofiber scaffold containing ethanol extract of pomegranate peel: Design, characterization and in vitro assay. Food and Bioprocess Technology, 14(5), 935–944.
Savaghebi, D., Ghaderi-Ghahfarokhi, M., & Barzegar, M. (2021). Encapsulation of Sargassum boveanum Algae extract in nano-liposomes: Application in functional mayonnaise production. Food and Bioprocess Technology, 1–15.
Sessa, M., Casazza, A. A., Perego, P., Tsao, R., Ferrari, G., & Donsì, F. (2013). Exploitation of polyphenolic extracts from grape marc as natural antioxidants by encapsulation in lipid-based nanodelivery systems. Food and Bioprocess Technology, 6(10), 2609–2620.
Souza, M. P., Vaz, A. F., Correia, M. T., Cerqueira, M. A., Vicente, A. A., & Carneiro-da-Cunha, M. G. (2014). Quercetin-loaded lecithin/chitosan nanoparticles for functional food applications. Food and Bioprocess Technology, 7(4), 1149–1159.
Shashidhar, G. M., & Manohar, B. (2018). Nanocharacterization of liposomes for the encapsulation of water soluble compounds from Cordyceps sinensis CS1197 by a supercritical gas anti-solvent technique. RSC Advances, 8(60), 34634–34649.
Tavares, L., Barros, H. L. B., Vaghetti, J. C. P., & Noreña, C. P. Z. (2019). Microencapsulation of garlic extract by complex coacervation using whey protein isolate/chitosan and gum Arabic/chitosan as wall materials: Influence of anionic biopolymers on the physicochemical and structural properties of microparticles. Food and Bioprocess Technology, 12(12), 2093–2106.
Tavassoli-Kafrani, E., Goli, S. A. H., & Fathi, M. (2018). Encapsulation of orange essential oil using cross-linked electrospun gelatin nanofibers. Food and Bioprocess Technology, 11(2), 427–434.
Ulkowski, M., Musialik, M., & Litwinienko, G. (2005). Use of differential scanning calorimetry to study lipid oxidation. 1. Oxidative stability of lecithin and linolenic acid. Journal of agricultural and food chemistry, 53(23), 9073–9077.
Weerakody, R., Fagan, P., & Kosaraju, S. L. (2008). Chitosan microspheres for encapsulation of α-lipoic acid. International Journal of Pharmaceutics, 357(1–2), 213–218.
Zhao, Y., Cai, F., Shen, X., & Su, H. (2020). A high stable pH-temperature dual-sensitive liposome for tuning anticancer drug release. Synthetic and Systems Biotechnology, 5(2), 103–110.
Zou, L. Q., Liu, W., Liu, W. L., Liang, R. H., Li, T., Liu, C. M., & Liu, Z. (2014). Characterization and bioavailability of tea polyphenol nanoliposome prepared by combining an ethanol injection method with dynamic high-pressure microfluidization. Journal of Agricultural and Food Chemistry, 62(4), 934–941.
Funding
This research was supported by the Sari Agricultural Sciences and Natural Resources University for analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gorjian, H., Raftani Amiri, Z., Mohammadzadeh Milani, J. et al. Influence of Nanovesicle Type, Nanoliposome and Nanoniosome, on Antioxidant and Antimicrobial Activities of Encapsulated Myrtle Extract: A Comparative Study. Food Bioprocess Technol 15, 144–164 (2022). https://doi.org/10.1007/s11947-021-02747-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-021-02747-3