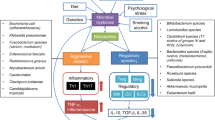
Opinion statement
Despite recent major strides in our understanding of the genetic and microbial influences that contribute to the development of the inflammatory bowel diseases (IBDs), their etiology continues to be enigmatic. Results from experiments in animal models of IBDs overwhelmingly support a causal role of the microbiota in these diseases, though whether such a cause-effect relationship exists in human IBDs is still uncertain. Therefore, virtually all currently approved and most often prescribed treatments for IBDs are directed toward the over-active immune response in these diseases rather than the intestinal bacteria. Nevertheless, there is an important need for non-immunosuppressive therapies that may present a more favorable risk-benefit profile such as those that selectively target the disruptions in gut microbiota that accompany IBDs. This need has led to clinical trials of various microbial-directed therapies including fecal microbial transplant, antibiotics, probiotics, and prebiotics. Unfortunately, these published studies, many of which are small, have generally failed to demonstrate a consistent benefit of these agents in IBDs, thus leading to slow acceptance of microbe-focused treatments for these conditions. In this article, we review and summarize the microbial basis for IBDs and the results of the most recent trials of fecal microbial transplant, antibiotics, probiotics, and prebiotics in IBDs. We also comment on possible safety concerns with these agents, speculate on why they have failed to show efficacy in certain clinical settings, and propose strategies to improve their usefulness.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kappelman MD, Rifas-Shiman SL, Porter CQ, Ollendorf DA, Sandler RS, Galanko JA et al (2008) Direct health care costs of Crohn’s disease and ulcerative colitis in us children and adults. Gastroenterology 135(6):1907–13
Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY et al (2012) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491(7422):119–24, The most comprehensive genome-wide association study in IBD to date. Identified 163 IBD susceptibility loci
Hansen J, Gulati A, Sartor RB (2010) The role of mucosal immunity and host genetics in defining intestinal commensal bacteria. Curr Opin Gastroenterol 26(6):564–71
Seekatz AM, Aas J, Gessert CE, Rubin TA, Saman DM, Bakken JS et al (2014) Recovery of the gut microbiome following fecal microbiota transplantation. MBio 5(3):e00893–14
Gevers D, Kugathasan S, Denson LA, Vazquez-Baeza Y, Van Treuren W, Ren B et al (2014) The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 15(3):382–92
Haberman Y, Tickle TL, Dexheimer PJ, Kim MO, Tang D, Karns R et al (2014) Pediatric crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J Clin Invest 124(8):3617–33, The largest prospective microbiome and transcriptome study in IBD tissues to date. Demonstrated disease-specific (CD vs. UC vs. healthy) transcriptional and microbial signatures in inflamed and non-inflamed intestinal tissues at the time of diagnosis
Ott SJ, Kuhbacher T, Musfeldt M, Rosenstiel P, Hellmig S, Rehman A et al (2008) Fungi and inflammatory bowel diseases: Alterations of composition and diversity. Scand J Gastroenterol 43(7):831–41
Li Q, Wang C, Tang C, He Q, Li N, Li J (2014) Dysbiosis of gut fungal microbiota is associated with mucosal inflammation in Crohn’s disease. J Clin Gastroenterol 48(6):513–23
Perez-Brocal V, Garcia-Lopez R, Vazquez-Castellanos JF, Nos P, Beltran B, Latorre A et al (2013) Study of the viral and microbial communities associated with Crohn’s disease: A metagenomic approach. Clin Transl Gastroenterol 4:e36
Wagner J, Maksimovic J, Farries G, Sim WH, Bishop RF, Cameron DJ et al (2013) Bacteriophages in gut samples from pediatric Crohn’s disease patients: Metagenomic analysis using 454 pyrosequencing. Inflamm Bowel Dis 19(8):1598–608
Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV et al (2012) Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 13(9):R79
van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM et al (2013) Duodenal infusion of donor feces for recurrent clostridium difficile. N Engl J Med 368(5):407–15, First published RCT demonstrating efficacy of FMT in C. difficile colitis
Bennet JD, Brinkman M (1989) Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet 1(8630):164
Kunde S, Pham A, Bonczyk S, Crumb T, Duba M, Conrad H Jr et al (2013) Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr 56(6):597–601
Suskind DL, Singh N, Nielson H, Wahbeh G. Fecal microbial transplant via nasogastric tube for active pediatric ulcerative colitis. J Pediatr Gastroenterol Nutr. 2014.
Kump PK, Grochenig HP, Lackner S, Trajanoski S, Reicht G, Hoffmann KM et al (2013) Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis 19(10):2155–65
Cui B, Feng Q, Wang H, Wang M, Peng Z, Li P, et al. Fecal microbiota transplantation through mid-gut for refractory Crohn’s disease: safety, feasibility and efficacy trial results. J Gastroenterol Hepatol. 2014.
Moayyedi P, Surette M, Wolfe M, Taraschi R, Kim P, Libertucci J et al (2014) A randomized, placebo controlled trial of fecal microbiota therapy in active ulcerative colitis. Gastroenterology 146(5):S-159, (Abstract). First results of a RCT to study the effect of FMT in IBD. Showed that UC patients who received FMT experienced a non-significant clinical improvement
Libertucci J, Whelan FJ, Moayyedi P, Lee CH, Wolfe M, Onishi C et al (2014) Investigating the microbiome pre and post fecal microbiota therapy from active ulcerative colitis patients in a randomized placebo controlled trial. Gastroenterology 146(5):S-902 (Abstract)
Bermejo F, Garrido E, Chaparro M, Gordillo J, Manosa M, Algaba A et al (2012) Efficacy of different therapeutic options for spontaneous abdominal abscesses in Crohn’s disease: Are antibiotics enough? Inflamm Bowel Dis 18(8):1509–14
Oshima T, Takesue Y, Ikeuchi H, Matsuoka H, Nakajima K, Uchino M et al (2013) Preoperative oral antibiotics and intravenous antimicrobial prophylaxis reduce the incidence of surgical site infections in patients with ulcerative colitis undergoing ipaa. Dis Colon Rectum 56(10):1149–55
Scribano ML, Prantera C (2013) Antibiotics and inflammatory bowel diseases. Dig Dis 31(3–4):379–84
Bernstein CN (2014) Antibiotics, probiotics and prebiotics in ibd. Nestle Nutr Inst Workshop Ser 79:83–100, A comprehensive review of manipulating the microbiome in IBD
Ford AC, Khan KJ, Achkar JP, Moayyedi P (2012) Efficacy of oral vs. Topical, or combined oral and topical 5-aminosalicylates, in ulcerative colitis: Systematic review and meta-analysis. Am J Gastroenterol 107(2):167–76, author reply 77
Wang SL, Wang ZR, Yang CQ (2012) Meta-analysis of broad-spectrum antibiotic therapy in patients with active inflammatory bowel disease. Exp Ther Med 4(6):1051–6
Sutherland L, Singleton J, Sessions J, Hanauer S, Krawitt E, Rankin G et al (1991) Double blind, placebo controlled trial of metronidazole in Crohn’s disease. Gut 32(9):1071–5
Colombel JF, Lemann M, Cassagnou M, Bouhnik Y, Duclos B, Dupas JL et al (1999) A controlled trial comparing ciprofloxacin with mesalazine for the treatment of active Crohn’s disease. Groupe d’etudes therapeutiques des affections inflammatoires digestives (getaid). Am J Gastroenterol 94(3):674–8
Arnold GL, Beaves MR, Pryjdun VO, Mook WJ (2002) Preliminary study of ciprofloxacin in active Crohn’s disease. Inflamm Bowel Dis 8(1):10–5
Steinhart AH, Feagan BG, Wong CJ, Vandervoort M, Mikolainis S, Croitoru K et al (2002) Combined budesonide and antibiotic therapy for active Crohn’s disease: A randomized controlled trial. Gastroenterology 123(1):33–40
Prantera C, Lochs H, Grimaldi M, Danese S, Scribano ML, Gionchetti P et al (2012) Rifaximin-extended intestinal release induces remission in patients with moderately active Crohn’s disease. Gastroenterology 142(3):473–81.e4, First published RCT of rifaximin in CD. Showed that rifaximin reduced clinical symptoms of CD in a dose-independent fashion
Selby W, Pavli P, Crotty B, Florin T, Radford-Smith G, Gibson P et al (2007) Two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for Crohn’s disease. Gastroenterology 132(7):2313–9
Brandt LJ, Bernstein LH, Boley SJ, Frank MS (1982) Metronidazole therapy for perineal Crohn’s disease: A follow-up study. Gastroenterology 83(2):383–7
Thia KT, Mahadevan U, Feagan BG, Wong C, Cockeram A, Bitton A et al (2009) Ciprofloxacin or metronidazole for the treatment of perianal fistulas in patients with Crohn’s disease: A randomized, double-blind, placebo-controlled pilot study. Inflamm Bowel Dis 15(1):17–24
West RL, van der Woude CJ, Hansen BE, Felt-Bersma RJ, van Tilburg AJ, Drapers JA et al (2004) Clinical and endosonographic effect of ciprofloxacin on the treatment of perianal fistulae in Crohn’s disease with infliximab: A double-blind placebo-controlled study. Aliment Pharmacol Ther 20(11–12):1329–36
Dewint P, Hansen BE, Verhey E, Oldenburg B, Hommes DW, Pierik M et al (2014) Adalimumab combined with ciprofloxacin is superior to adalimumab monotherapy in perianal fistula closure in Crohn’s disease: A randomised, double-blind, placebo controlled trial (adafi). Gut 63(2):292–9, A well-designed RCT showing the benefit of adding ciprofloxacin to anti-TNF treatment for perianal fistulae in CD
Dejaco C, Harrer M, Waldhoer T, Miehsler W, Vogelsang H, Reinisch W (2003) Antibiotics and azathioprine for the treatment of perianal fistulas in Crohn’s disease. Aliment Pharmacol Ther 18(11–12):1113–20
Rutgeerts P, Hiele M, Geboes K, Peeters M, Penninckx F, Aerts R et al (1995) Controlled trial of metronidazole treatment for prevention of Crohn’s recurrence after ileal resection. Gastroenterology 108(6):1617–21
Rutgeerts P, Van Assche G, Vermeire S, D’Haens G, Baert F, Noman M et al (2005) Ornidazole for prophylaxis of postoperative Crohn’s disease recurrence: A randomized, double-blind, placebo-controlled trial. Gastroenterology 128(4):856–61
D’Haens GR, Vermeire S, Van Assche G, Noman M, Aerden I, Van Olmen G et al (2008) Therapy of metronidazole with azathioprine to prevent postoperative recurrence of Crohn’s disease: A controlled randomized trial. Gastroenterology 135(4):1123–9
Manosa M, Cabre E, Bernal I, Esteve M, Garcia-Planella E, Ricart E et al (2013) Addition of metronidazole to azathioprine for the prevention of postoperative recurrence of Crohn’s disease: A randomized, double-blind, placebo-controlled trial. Inflamm Bowel Dis 19(9):1889–95
Herfarth HH, Katz JA, Hanauer SB, Sandborn WJ, Loftus EV Jr, Sands BE et al (2013) Ciprofloxacin for the prevention of postoperative recurrence in patients with Crohn’s disease: A randomized, double-blind, placebo-controlled pilot study. Inflamm Bowel Dis 19(5):1073–9
Sato K, Chiba T, Ohkusa T (2009) Serial changes of cytokines in active ulcerative colitis: Effects of antibiotic combination therapy. Hepatogastroenterology 56(93):1016–21
Mantzaris GJ, Hatzis A, Kontogiannis P, Triadaphyllou G (1994) Intravenous tobramycin and metronidazole as an adjunct to corticosteroids in acute, severe ulcerative colitis. Am J Gastroenterol 89(1):43–6
Mantzaris GJ, Petraki K, Archavlis E, Amberiadis P, Kourtessas D, Christidou A et al (2001) A prospective randomized controlled trial of intravenous ciprofloxacin as an adjunct to corticosteroids in acute, severe ulcerative colitis. Scand J Gastroenterol 36(9):971–4
Turunen UM, Farkkila MA, Hakala K, Seppala K, Sivonen A, Ogren M et al (1998) Long-term treatment of ulcerative colitis with ciprofloxacin: A prospective, double-blind, placebo-controlled study. Gastroenterology 115(5):1072–8
Ohkusa T, Kato K, Terao S, Chiba T, Mabe K, Murakami K et al (2010) Newly developed antibiotic combination therapy for ulcerative colitis: A double-blind placebo-controlled multicenter trial. Am J Gastroenterol 105(8):1820–9
Koido S, Ohkusa T, Kajiura T, Shinozaki J, Suzuki M, Saito K et al (2014) Long-term alteration of intestinal microbiota in patients with ulcerative colitis by antibiotic combination therapy. PLoS One 9(1):e86702
Turner D, Levine A, Kolho KL, Shaoul R, Ledder O (2014) Combination of oral antibiotics may be effective in severe pediatric ulcerative colitis: A preliminary report. J Crohns Colitis 8(11):1464–70
Gionchetti P, Rizzello F, Ferrieri A, Venturi A, Brignola C, Ferretti M et al (1999) Rifaximin in patients with moderate or severe ulcerative colitis refractory to steroid-treatment: A double-blind, placebo-controlled trial. Dig Dis Sci 44(6):1220–1
Chowdhry S, Katz JA (2014) Update on the pathogenesis and management of pouchitis. Curr Infect Dis Rep 16(12):442
Gionchetti P, Calafiore A, Riso D, Liguori G, Calabrese C, Vitali G et al (2012) The role of antibiotics and probiotics in pouchitis. Ann Gastroenterol 25(2):100–5
Shen B, Achkar JP, Lashner BA, Ormsby AH, Remzi FH, Brzezinski A et al (2001) A randomized clinical trial of ciprofloxacin and metronidazole to treat acute pouchitis. Inflamm Bowel Dis 7(4):301–5
Madden MV, McIntyre AS, Nicholls RJ (1994) Double-blind crossover trial of metronidazole versus placebo in chronic unremitting pouchitis. Dig Dis Sci 39(6):1193–6
Isaacs KL, Sandler RS, Abreu M, Picco MF, Hanauer SB, Bickston SJ et al (2007) Rifaximin for the treatment of active pouchitis: A randomized, double-blind, placebo-controlled pilot study. Inflamm Bowel Dis 13(10):1250–5
McLaughlin SD, Clark SK, Tekkis PP, Ciclitira PJ, Nicholls RJ (2011) An open study of maintenance antibiotic therapy for chronic antibiotic-dependent pouchitis: Efficacy, complications and outcome. Colorectal Dis 13(4):438–44
Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC et al (2002) Four-week open-label trial of metronidazole and ciprofloxacin for the treatment of recurrent or refractory pouchitis. Aliment Pharmacol Ther 16(5):909–17
Gionchetti P, Rizzello F, Venturi A, Ugolini F, Rossi M, Brigidi P et al (1999) Antibiotic combination therapy in patients with chronic, treatment-resistant pouchitis. Aliment Pharmacol Ther 13(6):713–8
Shen B, Fazio VW, Remzi FH, Bennett AE, Lopez R, Brzezinski A et al (2007) Combined ciprofloxacin and tinidazole therapy in the treatment of chronic refractory pouchitis. Dis Colon Rectum 50(4):498–508
Shen J, Zuo ZX, Mao AP (2014) Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn’s disease, and pouchitis: Meta-analysis of randomized controlled trials. Inflamm Bowel Dis 20(1):21–35
Kruis W (2013) Probiotics. Dig Dis 31(3–4):385–7
Naidoo K, Gordon M, Fagbemi AO, Thomas AG, Akobeng AK (2011) Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 12:CD007443
Kruis W, Fric P, Pokrotnieks J, Lukas M, Fixa B, Kascak M et al (2004) Maintaining remission of ulcerative colitis with the probiotic Escherichia coli nissle 1917 is as effective as with standard mesalazine. Gut 53(11):1617–23
Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT (1999) Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: A randomised trial. Lancet 354(9179):635–9
Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A (2009) Effect of a probiotic preparation (vsl#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol 104(2):437–43
Zocco MA, dal Verme LZ, Cremonini F, Piscaglia AC, Nista EC, Candelli M et al (2006) Efficacy of lactobacillus gg in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther 23(11):1567–74
Wildt S, Nordgaard I, Hansen U, Brockmann E, Rumessen JJ (2011) A randomised double-blind placebo-controlled trial with lactobacillus acidophilus la-5 and bifidobacterium animalis subsp. Lactis bb-12 for maintenance of remission in ulcerative colitis. J Crohns Colitis 5(2):115–21
Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P et al (2009) The probiotic preparation, vsl#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol 7(11):1202–9, 09 e1
Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM et al (2010) Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic vsl#3 as adjunctive to a standard pharmaceutical treatment: A double-blind, randomized, placebo-controlled study. Am J Gastroenterol 105(10):2218–27
Butterworth AD, Thomas AG, Akobeng AK (2008) Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 3:CD006634
Doherty GA, Bennett GC, Cheifetz AS, Moss AC (2010) Meta-analysis: Targeting the intestinal microbiota in prophylaxis for post-operative Crohn’s disease. Aliment Pharmacol Ther 31(8):802–9
Prantera C, Scribano ML, Falasco G, Andreoli A, Luzi C (2002) Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn’s disease: A randomised controlled trial with lactobacillus gg. Gut 51(3):405–9
Marteau P, Lemann M, Seksik P, Laharie D, Colombel JF, Bouhnik Y et al (2006) Ineffectiveness of lactobacillus johnsonii la1 for prophylaxis of postoperative recurrence in Crohn’s disease: A randomised, double blind, placebo controlled getaid trial. Gut 55(6):842–7
Van Gossum A, Dewit O, Louis E, de Hertogh G, Baert F, Fontaine F et al (2007) Multicenter randomized-controlled clinical trial of probiotics (lactobacillus johnsonii, la1) on early endoscopic recurrence of Crohn’s disease after lleo-caecal resection. Inflamm Bowel Dis 13(2):135–42
Fedorak RN, Feagan BG, Hotte N, Leddin D, Dieleman LA, Petrunia DM et al (2014) The probiotic VSL#3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn’s disease. Clin Gastoenterol Hepatol Nov 6. doi:10.1016/j.cgh.2014.10.031
Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ et al (2008) Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of crohn disease patients. Proc Natl Acad Sci U S A 105(43):16731–6
Steed H, Macfarlane GT, Blackett KL, Bahrami B, Reynolds N, Walsh SV et al (2010) Clinical trial: The microbiological and immunological effects of synbiotic consumption - a randomized double-blind placebo-controlled study in active Crohn’s disease. Aliment Pharmacol Ther 32(7):872–83
Gionchetti P, Calafiore A, Pratico C, Laureti S, Vitali G, Poggioli G et al (2012) Randomized controlled trials in pouchitis. Rev Recent Clin Trials 7(4):303–6
Shen B (2012) Acute and chronic pouchitis–pathogenesis, diagnosis and treatment. Nat Rev Gastroenterol Hepatol 9(6):323–33, Excellent review of pouchitis therapies by a leading authority in the field
Guslandi M (2014) Role of probiotics in the management of pouchitis. Curr Pharm Des 20(28):4561–4
Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G et al (2000) Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: A double-blind, placebo-controlled trial. Gastroenterology 119(2):305–9
Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC et al (2004) Once daily high dose probiotic therapy (vsl#3) for maintaining remission in recurrent or refractory pouchitis. Gut 53(1):108–14
Shen B, Brzezinski A, Fazio VW, Remzi FH, Achkar JP, Bennett AE et al (2005) Maintenance therapy with a probiotic in antibiotic-dependent pouchitis: Experience in clinical practice. Aliment Pharmacol Ther 22(8):721–8
Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P et al (2003) Prophylaxis of pouchitis onset with probiotic therapy: A double-blind, placebo-controlled trial. Gastroenterology 124(5):1202–9
Persborn M, Gerritsen J, Wallon C, Carlsson A, Akkermans LM, Soderholm JD (2013) The effects of probiotics on barrier function and mucosal pouch microbiota during maintenance treatment for severe pouchitis in patients with ulcerative colitis. Aliment Pharmacol Ther 38(7):772–83
Kuisma J, Mentula S, Jarvinen H, Kahri A, Saxelin M, Farkkila M (2003) Effect of lactobacillus rhamnosus gg on ileal pouch inflammation and microbial flora. Aliment Pharmacol Ther 17(4):509–15
Gionchetti P, Rizzello F, Morselli C, Poggioli G, Tambasco R, Calabrese C et al (2007) High-dose probiotics for the treatment of active pouchitis. Dis Colon Rectum 50(12):2075–82, discussion 82-4
Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H et al (2014) Activation of gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40(1):128–39
Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P et al (2013) Metabolites produced by commensal bacteria promote peripheral regulatory t-cell generation. Nature 504(7480):451–5, This study demonstrates that bacterial-derived short chain fatty acids can reduce inflammation by enhancing the function of regulatory T cells
Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D et al (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory t cells. Nature 504(7480):446–50, This study demonstrates that bacterial-derived short chain fatty acids can reduce inflammation by enhancing the function of regulatory T cells
Looijer-van Langen MA, Dieleman LA (2009) Prebiotics in chronic intestinal inflammation. Inflamm Bowel Dis 15(3):454–62
Verbeke KA, Boesmans L, Boets E (2014) Modulating the microbiota in inflammatory bowel diseases: Prebiotics, probiotics or faecal transplantation? Proc Nutr Soc 73(4):490–7
Benjamin JL, Hedin CR, Koutsoumpas A, Ng SC, McCarthy NE, Hart AL et al (2011) Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut 60(7):923–9
Casellas F, Borruel N, Torrejon A, Varela E, Antolin M, Guarner F et al (2007) Oral oligofructose-enriched inulin supplementation in acute ulcerative colitis is well tolerated and associated with lowered faecal calprotectin. Aliment Pharmacol Ther 25(9):1061–7
Fujimori S, Tatsuguchi A, Gudis K, Kishida T, Mitsui K, Ehara A et al (2007) High dose probiotic and prebiotic cotherapy for remission induction of active Crohn’s disease. J Gastroenterol Hepatol 22(8):1199–204
Chermesh I, Tamir A, Reshef R, Chowers Y, Suissa A, Katz D et al (2007) Failure of synbiotic 2000 to prevent postoperative recurrence of Crohn’s disease. Dig Dis Sci 52(2):385–9
Welters CF, Heineman E, Thunnissen FB, van den Bogaard AE, Soeters PB, Baeten CG (2002) Effect of dietary inulin supplementation on inflammation of pouch mucosa in patients with an ileal pouch-anal anastomosis. Dis Colon Rectum 45(5):621–7
Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI (2009) The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 1(6):6ra14
Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S et al (2010) Metabolic syndrome and altered gut microbiota in mice lacking toll-like receptor 5. Science 328(5975):228–31
Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T et al (2012) Inflammasome-mediated dysbiosis regulates progression of nafld and obesity. Nature 482(7384):179–85
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444(7122):1027–31
Santino I, Alari A, Bono S, Teti E, Marangi M, Bernardini A et al (2014) Saccharomyces cerevisiae fungemia, a possible consequence of the treatment of clostridium difficile colitis with a probioticum. Int J Immunopathol Pharmacol 27(1):143–6
Joossens M, De Preter V, Ballet V, Verbeke K, Rutgeerts P, Vermeire S (2012) Effect of oligofructose-enriched inulin (of-in) on bacterial composition and disease activity of patients with Crohn’s disease: Results from a double-blinded randomised controlled trial. Gut 61(6):958
Ungaro R, Bernstein CN, Gearry R, Hviid A, Kolho KL, Kronman MP et al (2014) Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: A meta-analysis. Am J Gastroenterol 109(11):1728–38
Shaw SY, Blanchard JF, Bernstein CN (2010) Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am J Gastroenterol 105(12):2687–92
Sokol H, Lay C, Seksik P, Tannock GW (2008) Analysis of bacterial bowel communities of ibd patients: What has it revealed? Inflamm Bowel Dis 14(6):858–67
Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA et al (2011) The toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332(6032):974–7
Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W et al (2000) Treatment of murine colitis by lactococcus lactis secreting interleukin-10. Science 289(5483):1352–5
Compliance with Ethics Guidelines
Conflict of Interest
Jonathan J. Hansen declares that he has no conflict of interest.
R. Balfour Sartor has received consulting fees/honorarium and paid travel accommodations from Dannon/Yakult and grants from Salix and GSK
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Inflammatory Bowel Disease
Rights and permissions
About this article
Cite this article
Hansen, J.J., Sartor, R.B. Therapeutic Manipulation of the Microbiome in IBD: Current Results and Future Approaches. Curr Treat Options Gastro 13, 105–120 (2015). https://doi.org/10.1007/s11938-014-0042-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-014-0042-7