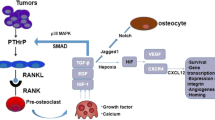
Abstract
Purpose of Review
Breast and prostate tumors frequently metastasize to the bone, but the underlying mechanisms for osteotropism remain elusive. An emerging feature of metastatic progression is metabolic adaptation of cancer cells to new environments. This review will summarize the recent advances on how cancer cells utilize amino acid metabolism during metastasis, from early dissemination to interactions with the bone microenvironment.
Recent Findings
Recent studies have suggested that certain metabolic preferences for amino acids may be associated with bone metastasis. Once in the bone microenvironment, cancer cells encounter a favorable microenvironment, where a changing nutrient composition of the tumor-bone microenvironment may alter metabolic interactions with bone-resident cells to further drive metastatic outgrowth.
Summary
Enhanced amino acid metabolic programs are associated with bone metastatic disease and may be further augmented by the bone microenvironment. Additional studies are necessary to fully elucidate the role of amino acid metabolism on bone metastasis.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–64. https://doi.org/10.1126/science.1203543.
Huang JF, Shen J, Li X, Rengan R, Silvestris N, Wang M, et al. Incidence of patients with bone metastases at diagnosis of solid tumors in adults: a large population-based study. Ann Transl Med. 2020;8:482–482. https://doi.org/10.21037/atm.2020.03.55.
Langley RR, Fidler IJ. The seed and soil hypothesis revisited—the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer. 2011;128:2527–35. https://doi.org/10.1002/ijc.26031.
Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science 2020:368:eaaw5473. https://doi.org/10.1126/science.aaw5473
Johnson RW, Suva LJ. Hallmarks of bone metastasis. Calcif Tissue Int. 2018;102:141–51. https://doi.org/10.1007/s00223-0170362-4.
Bergers G, Fendt SM. The metabolism of cancer cells during metastasis. Nat Rev Cancer. 2021;21:162–80. https://doi.org/10.1038/s41568-020-00320-2.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. https://doi.org/10.1016/j.cell.2011.02.013.
Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. https://doi.org/10.1016/j.cmet.2015.12.006.
Zanotelli MR, Goldblatt ZE, Miller JP, Bordeleau F, Li J, VanderBurgh JA, et al. Regulation of ATP utilization during metastatic cell migration by collagen architecture. Mol Biol Cell. 2018;29:1–9. https://doi.org/10.1091/mbc.E17-01-0041.
Yang L, Moss T, Mangala LS, Marini J, Zhao H, Wahlig S, et al. Metabolic shifts toward glutamine regulate tumor growth, invasion and bioenergetics in ovarian cancer. Mol Syst Biol. 2014;10:728. https://doi.org/10.1002/msb.20134892.
Rodrigues MF, Obre E, De Melo FHM, Santos GC, Galina A, Jasiulionis MG, et al. Enhanced OXPHOS, glutaminolysis and β-oxidation constitute the metastatic phenotype of melanoma cells. Biochem J. 2016;473:703–15. https://doi.org/10.1042/BJ20150645.
Xu J, Zhang J, Li L, Mao J, You T, Li Y. SOX12 expression is associated with progression and poor prognosis in human breast cancer. Am J Transl Res. 2020;12:8162.
Du F, Chen J, Liu H, Cai Y, Cao T, Han W, et al. SOX12 promotes colorectal cancer cell proliferation and metastasis by regulating asparagine synthesis. Cell Death Dis. 2019;10:239. https://doi.org/10.1038/s41419-019-1481-9.
Knott SRV, Wagenblast E, Khan S, Kim SY, Soto M, Wagner M, et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature. 2018;554:378–81. https://doi.org/10.1038/nature25465.
• Soflaee MH, Kesavan R, Sahu U, Tasdogan A, Villa E, Djabari Z, et al. Purine nucleotide depletion prompts cell migration by stimulating the serine synthesis pathway. Nat Commun. 2022;13:2698. https://doi.org/10.1038/s41467-022-30362-z. Demonstrates that nucleotide stress in the primary tumor increases serine biosynthetic pathways and EMT to promote metastasis.
Jin H, Qiao F, Chen L, Lu C, Xu L, Gao X. Serum metabolomic signatures of lymph node metastasis of esophageal squamous cell carcinoma. J Proteome Res. 2014;13:4091–103. https://doi.org/10.1021/pr500483z.
Jin L, Chun J, Pan C, Kumar A, Zhang G, Ha Y, et al. The PLAG1-GDH1 axis promotes anoikis resistance and tumor metastasis through CamKK2-AMPK signaling in LKB1-deficient lung cancer. Mol Cell. 2018;69:87-99.e7. https://doi.org/10.1016/j.molcel.2017.11.025.
Zhang L, Yao Y, Zhang S, Liu Y, Guo H, Ahmed M, et al. Metabolic reprogramming toward oxidative phosphorylation identifies a therapeutic target for mantle cell lymphoma. Sci Transl Med. 2019;11:eaau1167. https://doi.org/10.1126/scitranslmed.aau1167.
Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–11. https://doi.org/10.1158/0008-5472.CAN-09-2994.
Lamb R, Ozsvari B, Lisanti CL, Tanowitz HB, Howell A, Martinez-Outschoorn UE, et al. Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types. Oncotarget. 2015;6:4569–84. https://doi.org/10.18632/oncotarget.3174.
Tan AS, Baty JW, Berridge MV. The role of mitochondrial electron transport in tumorigenesis and metastasis. Biochim Biophys Acta Gen Subj. 2014;1840:1454–63. https://doi.org/10.1016/j.bbagen.2013.10.016.
Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–8. https://doi.org/10.1073/pnas.0510511103.
Wang Q, Hardie RA, Hoy AJ, Van Geldermalsen M, Gao D, Fazli L, et al. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J Pathol. 2015;236:278–89. https://doi.org/10.1002/PATH.4518.
Edwards DN, Ngwa VM, Raybuck AL, Wang S, Hwang Y, Kim LC, et al. Selective glutamine metabolism inhibition in tumor cells improves antitumor T lymphocyte activity in triple-negative breast cancer. J Clin Invest. 2021;131:e140100. https://doi.org/10.1172/jci140100.
Ren L, Ruiz-Rodado V, Dowdy T, Huang S, Issaq SH, Beck J, et al. Glutaminase-1 (GLS1) inhibition limits metastatic progression in osteosarcoma. Cancer Metab. 2020;8:4. https://doi.org/10.1186/s40170-020-0209-8.
Elia I, Broekaert D, Christen S, Boon R, Radaelli E, Orth MF, et al. Proline metabolism supports metastasis formation and could be inhibited to selectively target metastasizing cancer cells. Nat Commun. 2017;8:15267. https://doi.org/10.1038/ncomms15267.
Rinaldi G, Pranzini E, Van Elsen J, Broekaert D, Funk CM, Planque M, et al. In vivo evidence for serine biosynthesis-defined sensitivity of lung metastasis, but not of primary breast tumors, to mTORC1 inhibition. Mol Cell. 2021;81:386-397.e7. https://doi.org/10.1016/j.molcel.2020.11.027.
•• Whitburn J, Rao SR, Morris E V., Tabata S, Hirayama A, Soga T, et al. Metabolic profiling of prostate cancer in skeletal microenvironments identifies G6PD as a key mediator of growth and survival. Sci Adv 2022:8:eabf9096. https://doi.org/10.1126/sciadv.abf9096Demonstrates that prostate cancer bone metastases are more metabolically active than primary tumors.
Dupuy F, Tabariès S, Andrzejewski S, Dong Z, Blagih J, Annis MG, et al. PDK1-Dependent metabolic reprogramming dictates metastatic potential in breast cancer. Cell Metab. 2015;22:577–89. https://doi.org/10.1016/J.CMET.2015.08.007.
Tandon M, Othman AH, Winogradzki M, Pratap J. Bone metastatic breast cancer cells display downregulation of PKC-ζ with enhanced glutamine metabolism. Gene. 2021;775:145419. https://doi.org/10.1016/j.gene.2021.145419.
Pollari S, Käkönen SM, Edgren H, Wolf M, Kohonen P, Sara H, et al. Enhanced serine production by bone metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res Treat. 2011;125:421–30. https://doi.org/10.1007/s10549-010-0848-5.
Jekabsons MB, Merrell M, Skubiz AG, Thornton N, Milasta S, Green D, et al. Breast cancer cells that preferentially metastasize to lung or bone are more glycolytic, synthesize serine at greater rates, and consume less ATP and NADPH than parent MDA-MB-231 cells. Cancer Metab. 2023;11:4. https://doi.org/10.1186/s40170-023-00303-5.
Li AM, Ducker GS, Li Y, Seoane JA, Xiao Y, Melemenidis S, et al. Metabolic profiling reveals a dependency of human metastatic breast cancer on mitochondrial serine and one-carbon unit metabolism. Mol Cancer Res. 2022;18:599–611. https://doi.org/10.1158/1541-7786.MCR-19-0606.
Rathore R, Caldwell KE, Schutt C, Brashears CB, Prudner BC, Ehrhardt WR, et al. Metabolic compensation activates pro-survival mTORC1 signaling upon 3-phosphoglycerate dehydrogenase inhibition in osteosarcoma. Cell Rep. 2021;34:108678. https://doi.org/10.1016/j.celrep.2020.108678.
Park HS, Chun YJ, Kim HS, Kim JH, Lee CK, Beom SH, et al. Clinical features and KRAS mutation in colorectal cancer with bone metastasis. Sci Rep. 2020;10:21180. https://doi.org/10.1038/s41598-020-78253-x.
Weng CC, Ding PY, Liu YH, Hawse JR, Subramaniam M, Wu CC, et al. Mutant Kras-induced upregulation of CD24 enhances prostate cancer stemness and bone metastasis. Oncogene. 2019;38:2005–19. https://doi.org/10.1038/s41388-018-0575-7.
Arriaga JM, Panja S, Alshalalfa M, Zhao J, Zou M, Giacobbe A, et al. A MYC and RAS co-activation signature in localized prostate cancer drives bone metastasis and castration resistance. Nat Cancer. 2020;1:1082–96. https://doi.org/10.1038/s43018-020-00125-0.
Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–5. https://doi.org/10.1038/nature12040.
Toda K, Nishikawa G, Iwamoto M, Itatani Y, Takahashi R, Sakai Y, et al. Clinical role of ASCT2 (SLC1A5) in KRAS-mutated colorectal cancer. Int J Mol Sci. 2017;18:1632. https://doi.org/10.3390/ijms18081632.
Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105:18782–7. https://doi.org/10.1073/pnas.0810199105.
Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–5. https://doi.org/10.1038/nature07823.
Sun L, Song L, Wan Q, Wu G, Li X, Wang Y, et al. cMyc-mediated activation of serine biosynthesis pathway is critical for cancer progression under nutrient deprivation conditions. Cell Res. 2015;25:429–44. https://doi.org/10.1038/cr.2015.33.
Liu W, Le A, Hancock C, Lane AN, Dang CV, Fan TWM, et al. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc Natl Acad Sci U S A. 2012;109:8983–8. https://doi.org/10.1073/pnas.1203244109.
Craze ML, Cheung H, Jewa N, Coimbra NDM, Soria D, El-Ansari R, et al. MYC regulation of glutamine–proline regulatory axis is key in luminal B breast cancer. Br J Cancer. 2018;118:258–65. https://doi.org/10.1038/bjc.2017.387.
Venkateswaran N, Lafita-Navarro MC, Hao YH, Kilgore JA, Perez-Castro L, Braverman J, et al. MYC promotes tryptophan uptake and metabolism by the kynurenine pathway in colon cancer. Genes Dev. 2019;33:1236–51. https://doi.org/10.1101/gad.327056.119.
Duan Z, Lu J. Involvement of aryl hydrocarbon receptor in L-kynurenine-mediated parathyroid hormone–related peptide expression. Horm Cancer. 2019;10:89–96. https://doi.org/10.1007/s12672-019-0357-x.
Vaught DB, Merkel AR, Lynch CC, Edwards J, Tantawy MN, Hilliard T, et al. EphA2 is a clinically relevant target for breast cancer bone metastatic disease. JBMR Plus. 2021;5:e10465. https://doi.org/10.1002/JBM4.10465.
Youngblood VM, Kim LC, Edwards DN, Hwang Y, Santapuram PR, Stirdivant SM, et al. The ephrin-A1/EPHA2 signaling axis regulates glutamine metabolism in HER2-positive breast cancer. Cancer Res. 2016;76:1825–36. https://doi.org/10.1158/0008-5472.CAN-15-0847.
Edwards DN, Ngwa VM, Wang S, Shiuan E, Brantley-Sieders DM, Kim LC, et al. The receptor tyrosine kinase EphA2 promotes glutamine metabolism in tumors by activating the transcriptional coactivators YAP and TAZ. Sci Signal. 2017;10:eaan4667. https://doi.org/10.1126/scisignal.aan4667.
Li C, Wang S, Xing Z, Lin A, Liang K, Song J, et al. A ROR1–HER3–lncRNA signalling axis modulates the Hippo–YAP pathway to regulate bone metastasis. Nat Cell Biol. 2017;19:106–19. https://doi.org/10.1038/ncb3464.
Zhou TY, Zhou YL, Qian MJ, Fang YZ, Ye S, Xin WX, et al. Interleukin-6 induced by YAP in hepatocellular carcinoma cells recruits tumor-associated macrophages. J Pharmacol Sci. 2018;138:89–95. https://doi.org/10.1016/j.jphs.2018.07.013.
Wang J, Filippakis H, Hougard T, Du H, Ye C, Liu HJ, et al. Interleukin-6 mediates PSAT1 expression and serine metabolism in TSC2-deficient cells. Proc Natl Acad Sci U S A. 2021;118:e2101268118. https://doi.org/10.1073/pnas.2101268118.
Song W, Hwang Y, Youngblood VM, Cook RS, Balko JM, Chen J, et al. Targeting EphA2 impairs cell cycle progression and growth of basal-like/triple-negative breast cancers. Oncogene. 2017;36:5620–30. https://doi.org/10.1038/onc.2017.170.
Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269–73. https://doi.org/10.1038/nature13034.
Knowles HJ, Athanasou NA. Acute hypoxia and osteoclast activity: a balance between enhanced resorption and increased apoptosis. J Pathol. 2009;218:256–64. https://doi.org/10.1002/path.2534.
Ma Z, Yu R, Zhao J, Sun L, Jian L, Li C, et al. Constant hypoxia inhibits osteoclast differentiation and bone resorption by regulating phosphorylation of JNK and IκBα. Inflamm Res. 2019;68:157–66. https://doi.org/10.1007/s00011-018-1209-9.
Rankin EB, Wu C, Khatri R, Wilson TLS, Andersen R, Araldi E, et al. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell. 2012;149:63–74. https://doi.org/10.1016/j.cell.2012.01.051.
Wu C, Rankin EB, Castellini L, Fernandez-Alcudia J, LaGory EL, Andersen R, et al. Oxygen-sensing PHDs regulate bone homeostasis through the modulation of osteoprotegerin. Genes Dev. 2015;29:817–31. https://doi.org/10.1101/gad.255000.114.
Rezaei A, Li Y, Turmaine M, Bertazzo S, Howard CA, Arnett TR, et al. Hypoxia mimetics restore bone biomineralisation in hyperglycaemic environments. Sci Rep. 2022;12:13944. https://doi.org/10.1038/s41598-022-18067-1.
Phadke PA, Mercer RR, Harms JF, Jia Y, Frost AR, Jewell JL, et al. Kinetics of metastatic breast cancer cell trafficking in bone. Clin Cancer Res. 2006;12:1431–40. https://doi.org/10.1158/1078-0432.CCR-05-1806.
Wang H, Yu C, Gao X, Welte T, Muscarella AM, Tian L, et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell. 2015;27:193–210. https://doi.org/10.1016/j.ccell.2014.11.017.
Devignes CS, Aslan Y, Brenot A, Devillers A, Schepers K, Fabre S, et al. HIF signaling in osteoblast-lineage cells promotes systemic breast cancer growth and metastasis in mice. Proc Natl Acad Sci U S A. 2018;115:E992–1001. https://doi.org/10.1073/pnas.1718009115.
Huang Z, Li G, Zhang Z, Gu R, Wang W, Lai X, et al. β2AR-HIF-1α-CXCL12 signaling of osteoblasts activated by isoproterenol promotes migration and invasion of prostate cancer cells. BMC Cancer. 2019;19:1142. https://doi.org/10.1186/s12885-019-6301-1.
Lu X, Yan CH, Yuan M, Wei Y, Hu G, Kang Y. In vivo dynamics and distinct functions of hypoxia in primary tumor growth and organotropic metastasis of breast cancer. Cancer Res. 2010;70:3905–14. https://doi.org/10.1158/0008-5472.CAN-09-3739.
Dunn LK, Mohammad KS, Fournier PGJ, McKenna CR, Davis HW, Niewolna M, et al. Hypoxia and TGF-β drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS One. 2009;4:e6896. https://doi.org/10.1371/journal.pone.0006896.
Hiraga T, Kizaka-Kondoh S, Hirota K, Hiraoka M, Yoneda T. Hypoxia and hypoxia-inducible factor-1 expression enhance osteolytic bone metastases of breast cancer. Cancer Res. 2007;67:4157–63. https://doi.org/10.1158/0008-5472.CAN-06-2355.
Todd VM, Vecchi LA, Clements ME, Snow KP, Ontko CD, Himmel L, et al. Hypoxia inducible factor signaling in breast tumors controls spontaneous tumor dissemination in a site-specific manner. Commun Biol. 2021;4:1122. https://doi.org/10.1038/s42003-021-02648-3.
Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–97. https://doi.org/10.1016/j.cmet.2006.01.012.
Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481:380–4. https://doi.org/10.1038/nature10602.
Xiang L, Mou J, Shao B, Wei Y, Liang H, Takano N, et al. Glutaminase 1 expression in colorectal cancer cells is induced by hypoxia and required for tumor growth, invasion, and metastatic colonization. Cell Death Dis. 2019;10:40. https://doi.org/10.1038/s41419-018-1291-5.
Hou KL, Lin SK, Kok SH, Wang HW, Lai EHH, Hong CY, et al. Increased expression of glutaminase in osteoblasts promotes macrophage recruitment in periapical lesions. J Endod. 2017;43:602–8. https://doi.org/10.1016/j.joen.2016.11.005.
Morten KJ, Badder L, Knowles HJ. Differential regulation of HIF-mediated pathways increases mitochondrial metabolism and ATP production in hypoxic osteoclasts. J Pathol. 2013;229:755–64. https://doi.org/10.1002/path.4159.
Thysell E, Surowiec I, Hörnberg E, Crnalic S, Widmark A, Johansson AI, et al. Metabolomic characterization of human prostate cancer bone metastases reveals increased levels of cholesterol. PLoS One. 2010;5:e14175. https://doi.org/10.1371/journal.pone.0014175.
•• Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature. 2021;593:282–8. https://doi.org/10.1038/s41586-021-03442-1. Demonstrates that cancer cells take up more glutamine than microenvironmental immune cells.
Leone RD, Zhao L, Englert JM, Sun IM, Oh MH, Sun IH, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366:1013–21. https://doi.org/10.1126/science.aav2588.
Chiu M, Toscani D, Marchica V, Taurino G, Costa F, Bianchi MG, et al. Myeloma cells deplete bone marrow glutamine and inhibit osteoblast differentiation limiting asparagine availability. Cancers. 2020;12:3267. https://doi.org/10.3390/cancers12113267.
Bolzoni M, Chiu M, Accardi F, Vescovini R, Airoldi I, Storti P, et al. Dependence on glutamine uptake and glutamine addiction characterize myeloma cells: a new attractive target. Blood. 2016;128:667–79. https://doi.org/10.1182/blood-2016-01-690743.
Triplett TA, Garrison KC, Marshall N, Donkor M, Blazeck J, Lamb C, et al. Reversal of indoleamine 2,3-dioxygenase–mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat Biotechnol. 2018;36:758–64. https://doi.org/10.1038/nbt.4180.
Yu Y, Newman H, Shen L, Sharma D, Hu G, Mirando AJ, et al. Glutamine metabolism regulates proliferation and lineage allocation in skeletal stem cells. Cell Metab. 2019;29:966-978.e4. https://doi.org/10.1016/j.cmet.2019.01.016.
Shen L, Sharma D, Yu Y, Long F, Karner CM. Biphasic regulation of glutamine consumption by WNT during osteoblast differentiation. J Cell Sci. 2021;134:jcs251645. https://doi.org/10.1242/jcs.251645.
• Sharma D, Yu Y, Shen L, Zhang GF, Karner CM. Slc1a5 provides glutamine and asparagine necessary for bone development in mice. Elife. 2021;10:e71595. https://doi.org/10.7554/eLife.71595. ()
Stegen S, Devignes CS, Torrekens S, Van Looveren R, Carmeliet P, Carmeliet G. Glutamine metabolism in osteoprogenitors is required for bone mass accrual and PTH-induced bone anabolism in male mice. J Bone Miner Res. 2021;36:604–16. https://doi.org/10.1002/jbmr.4219.
Karner CM, Esen E, Okunade AL, Patterson BW, Long F. Increased glutamine catabolism mediates bone anabolism in response to WNT signaling. J Clin Invest. 2015;125:551–62. https://doi.org/10.1172/JCI78470.
• Shen L, Yu Y, Zhou Y, Pruett-Miller SM, Zhang GF, Karner CM. SLC38A2 provides proline to fulfil unique synthetic demands arising during osteoblast differentiation and bone formation. Elife. 2022;11:e76963. https://doi.org/10.7554/eLife.76963. ()
Indo Y, Takeshita S, Ishii KA, Hoshii T, Aburatani H, Hirao A, et al. Metabolic regulation of osteoclast differentiation and function. J Bone Miner Res. 2013;28:2392–9. https://doi.org/10.1002/jbmr.1976.
Taubmann J, Krishnacoumar B, Böhm C, Faas M, Müller DIH, Adam S, et al. Metabolic reprogramming of osteoclasts represents a therapeutic target during the treatment of osteoporosis. Sci Rep. 2020;10:21020. https://doi.org/10.1038/s41598-020-77892-4.
Lee S, Kim HS, Kim MJ, Min KY, Choi WS, You JS. Glutamine metabolite α-ketoglutarate acts as an epigenetic co-factor to interfere with osteoclast differentiation. Bone. 2021;145:115836. https://doi.org/10.1016/J.BONE.2020.115836.
Tian J, Bao X, Yang F, Tang X, Jiang Q, Li Y, et al. Elevation of intracellular alpha-ketoglutarate levels inhibits osteoclastogenesis by suppressing the NF-κB signaling pathway in a PHD1-dependent manner. Nutrients. 2023;15:701. https://doi.org/10.3390/nu15030701.
Wang Y, Deng P, Liu Y, Wu Y, Chen Y, Guo Y, et al. Alpha-ketoglutarate ameliorates age-related osteoporosis via regulating histone methylations. Nat Commun. 2020;11:5596. https://doi.org/10.1038/s41467-020-19360-1.
Tran TQ, Lowman XH, Kong M. Molecular pathways: metabolic control of histone methylation and gene expression in cancer. Clin Cancer Res. 2017;23:4004–9. https://doi.org/10.1158/1078-0432.CCR-16-2506.
Arellano DL, Juárez P, Verdugo-Meza A, Almeida-Luna PS, Corral-Avila JA, Drescher F, et al. Bone microenvironment-suppressed T cells increase osteoclast formation and osteolytic bone metastases in mice. J Bone Miner Res. 2022;37:1446–63. https://doi.org/10.1002/jbmr.4615.
• Go M, Shin E, Jang SY, Nam M, Hwang GS, Lee SY. BCAT1 promotes osteoclast maturation by regulating branched-chain amino acid metabolism. Exp Mol Med. 2022;54:825–33. https://doi.org/10.1038/s12276-022-00775-3. Demonstrates that RANKL-induced osteoclast activation increases BCAA uptake and BCAT1 activity.
Pierce JL, Roberts RL, Yu K, Kendall RK, Kaiser H, Davis C, et al. Kynurenine suppresses osteoblastic cell energetics in vitro and osteoblast numbers in vivo. Exp Gerontol. 2020;130:110818. https://doi.org/10.1016/j.exger.2019.110818.
Eisa NH, Reddy SV, Elmansi AM, Kondrikova G, Kondrikov D, Shi XM, et al. Kynurenine promotes RANKL-induced osteoclastogenesis in vitro by activating the aryl hydrocarbon receptor pathway. Int J Mol Sci. 2020;21:7931. https://doi.org/10.3390/ijms21217931.
Zheng XQ, Wu YH, Huang JF, Wu AM. Neurophysiological mechanisms of cancer-induced bone pain. J Adv Res. 2022;35:117–27.
Pereira V, Goudet C. Emerging trends in pain modulation by metabotropic glutamate receptors. Front Mol Neurosci. 2019;11:464. https://doi.org/10.3389/fnmol.2018.00464.
Slosky LM, BassiriRad NM, Symons AM, Thompson M, Doyle T, Forte BL, et al. The cystine/glutamate antiporter system xc− drives breast tumor cell glutamate release and cancer-induced bone pain. Pain. 2016;157:2605–16. https://doi.org/10.1097/j.pain.0000000000000681.
Dornier E, Rabas N, Mitchell L, Novo D, Dhayade S, Marco S, et al. Glutaminolysis drives membrane trafficking to promote invasiveness of breast cancer cells. Nature Commun. 2017;8:1–14. https://doi.org/10.1038/s41467-017-02101-2.
Ungard RG, Linher-Melville K, Nashed M, Sharma M, Wen J, Singh G. xCT knockdown in human breast cancer cells delays onset of cancer-induced bone pain. Mol Pain. 2019;15:1744806918822185. https://doi.org/10.1177/1744806918822185.
Ungard RG, Seidlitz EP, Singh G. Inhibition of breast cancer-cell glutamate release with sulfasalazine limits cancer-induced bone pain. Pain. 2014;155:28–36. https://doi.org/10.1016/J.PAIN.2013.08.030.
Zhu YF, Linher-Melville K, Wu J, Fazzari J, Miladinovic T, Ungard R, et al. Bone cancer-induced pain is associated with glutamate signalling in peripheral sensory neurons. Mol Pain. 2020;16:1744806920911536. https://doi.org/10.1177/1744806920911536.
Fazzari J, Lin H, Murphy C, Ungard R, Singh G. Inhibitors of glutamate release from breast cancer cells; new targets for cancer-induced bone-pain. Sci Rep. 2015;5:8380. https://doi.org/10.1038/srep08380.
Lemberg KM, Gori SS, Tsukamoto T, Rais R, Slusher BS. Clinical development of metabolic inhibitors for oncology. J Clin Invest. 2022;132:e148550. https://doi.org/10.1172/JCI148550.
Stine ZE, Schug ZT, Salvino JM, Dang CV. Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discov. 2022;21:141–62. https://doi.org/10.1038/s41573-021-00339-6.
Muhammad N, Lee HM, Kim J. Oncology therapeutics targeting the metabolism of amino acids. Cells. 1904;2020:9. https://doi.org/10.3390/cells9081904.
Meric-Bernstam F, Tannir NM, Iliopoulos O, Lee RJ, Telli ML, Fan AC, et al. Telaglenastat plus cabozantinib or everolimus for advanced or metastatic renal cell carcinoma: an open-label phase I trial. Clin Cancer Res. 2022;28:1540–8. https://doi.org/10.1158/1078-0432.CCR-21-2972.
Ngo B, Kim E, Osorio-Vasquez V, Doll S, Bustraan S, Liang RJ, et al. Limited environmental serine and glycine confer brain metastasis sensitivity to PHGDH Inhibition. Cancer Discov. 2020;10:1352–73. https://doi.org/10.1158/2159-8290.CD-19-1228.
Marescal O, Cheeseman IM. Cellular mechanisms and regulation of quiescence. Dev Cell. 2020;55:259–71. https://doi.org/10.1016/j.devcel.2020.09.029.
Funding
This manuscript was supported by a Department of Defense CDMRP Award W81XWH2210109 (DNE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no conflicts of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Edwards, D.N. Amino Acid Metabolism in Bone Metastatic Disease. Curr Osteoporos Rep 21, 344–353 (2023). https://doi.org/10.1007/s11914-023-00797-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-023-00797-4