Abstract
Background
Osteoclasts are responsible for the bone loss in rheumatoid arthritis (RA). Hypoxia has been suggested to play key roles in pathological bone loss. However, the current understanding of the effects of hypoxia on osteoclastogenesis is controversial. Effects of hypoxia on both the formation and function of osteoclasts requires examination. In the current study, we aimed to explore the effect of hypoxia on osteoclast differentiation and the underlying mechanisms.
Methods
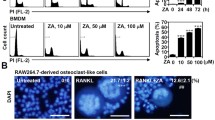
RAW264.7 cells and murine bone-marrow-derived monocytes were used to induce osteoclastogenesis in the presence of macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa B ligand (RANKL). Hypoxic conditions were maintained in a hypoxic chamber at 5% CO2 and 1% O2, balanced with N2. Osteoclasts were detected by tartrate-resistant acid phosphatase (TRAP) staining. A bone resorption assay was carried out in vitro using bone slices. RT-PCR was conducted to detect osteoclast markers and transcription factors. The phosphorylation of nuclear factor-κBα (IκBα), c-Jun N-terminal kinase (JNK), extracellular regulated protein kinase (ERK), and p38 was detected by western blotting. Mann–Whitney U test or Student’s t test was used to compare differences between the two groups.
Results
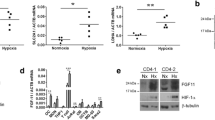
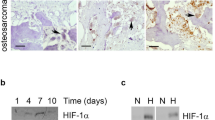
TRAP staining and the bone resorption assay revealed that hypoxia-restrained osteoclast differentiation and bone resorption. Expression of osteoclast markers including cathepsin K, RANK, and TRAP decreased during osteoclast differentiation under hypoxic conditions (all P < 0.05). Hypoxia at 1% O2 did not affect cell viability, whereas it dramatically abated RANKL-dependent phosphorylation of the JNK-mitogen-activated protein kinases (MAPK) and IκBα pathways. Moreover, the expression of nuclear factor of activated T-cell cytoplasmic 1 (NFATc1) was inhibited under hypoxic conditions (all P < 0.05).
Conclusions
These results suggest that constant hypoxia at 1% O2 significantly restrains osteoclast formation and resorbing function without affecting cell viability. Constant hypoxia might inhibit RANKL-induced osteoclastogenesis by regulating NFATc1 expression via interfering the phosphorylation of JNK and IκBα.






Similar content being viewed by others
References
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. https://doi.org/10.1038/nrdp.2018.1.
Li ZG. Facing the challenge of low recognition and high disability in rheumatoid arthritis in Chinese. Natl Med J Chin. 2009;89:1873–5.
Ji J, Zhang L, Zhang Q, Yin R, Fu T, Li L, et al. Functional disability associated with disease and quality-of-life parameters in Chinese patients with rheumatoid arthritis. Health Qual Life Outcomes. 2017;15:89. https://doi.org/10.1186/s12955-017-0659-z.
Tanaka Y, Ohira T. Mechanisms and therapeutic targets for bone damage in rheumatoid arthritis, in particular the RANK-RANKL system. Curr Opin Pharmacol. 2018;40:110–9. https://doi.org/10.1016/j.coph.2018.03.006.
Maurizi A, Rucci N. The osteoclast in bone metastasis: player and target. Cancers Basel. 2018. https://doi.org/10.3390/cancers10070218.
Ugay L, Kochetkova E, Nevzorova V, Maistrovskaia Y. Role of osteoprotegerin and receptor activator of nuclear factor-kappaB ligand in bone loss related to advanced chronic obstructive pulmonary disease. Chin Med J Engl. 2016;129:1696–703. https://doi.org/10.4103/0366-6999.185857.
Wang RY, Yang SH, Xu WH. Role of epithelium sodium channel in bone formation. Chin Med J. 2016;129:594–600. https://doi.org/10.4103/0366-6999.176994.
Amarasekara DS, Yun H, Kim S, Lee N, Kim H, Rho J. Regulation of osteoclast differentiation by cytokine networks. Immun Netw. 2018;18:e8. https://doi.org/10.4110/in.2018.18.e8.
Nanke Y, Kobashigawa T, Yago T, Kawamoto M, Yamanaka H, Kotake S. RANK expression and osteoclastogenesis in human monocytes in peripheral blood from rheumatoid arthritis patients. Biomed Res Int. 2016;2016:4874195. https://doi.org/10.1155/2016/4874195.
An J, Hao D, Zhang Q, Chen B, Zhang R, Wang Y, et al. Natural products for treatment of bone erosive diseases: the effects and mechanisms on inhibiting osteoclastogenesis and bone resorption. Int Immunopharmacol. 2016;36:118–31. https://doi.org/10.1016/j.intimp.2016.04.024.
Ye H, Arron JR, Lamothe B, Cirilli M, Kobayashi T, Shevde NK, et al. Distinct molecular mechanism for initiating TRAF6 signalling. Nature. 2002;418:443–7. https://doi.org/10.1038/nature00888.
Li L, Sapkota M, Gao M, Choi H, Soh Y. Macrolactin F inhibits RANKL-mediated osteoclastogenesis by suppressing Akt, MAPK and NFATc1 pathways and promotes osteoblastogenesis through a BMP-2/smad /Akt/Runx2 signaling pathway. Eur J Pharmacol. 2017;815:202–9. https://doi.org/10.1016/j.ejphar.2017.09.015.
Oh JH, Lee NK. Up-regulation of RANK expression via ERK1/2 by insulin contributes to the enhancement of osteoclast differentiation. Mol Cells. 2017;40:371–7. https://doi.org/10.14348/molcells.2017.0025.
Cong Q, Jia H, Li P, Qiu S, Yeh J, Wang Y, et al. p38alpha MAPK regulates proliferation and differentiation of osteoclast progenitors and bone remodeling in an aging-dependent manner. Sci Rep. 2017;7:45964. https://doi.org/10.1038/srep45964.
Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol 2014;5:511. https://doi.org/10.3389/fimmu.2014.00511.
Park JH, Lee NK, Lee SY. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol Cells. 2017;40:706–13. https://doi.org/10.14348/molcells.2017.0225.
Kim H, Choi HK, Shin JH, Kim KH, Huh JY, Lee SA, et al. Selective inhibition of RANK blocks osteoclast maturation and function and prevents bone loss in mice. J Clin Invest. 2009;119:813–25. https://doi.org/10.1172/JCI36809.
Shinohara M, Takayanagi H. Analysis of NFATc1-centered transcription factor regulatory networks in osteoclast formation. Methods Mol Biol. 2014;1164:171–6. https://doi.org/10.1007/978-1-4939-0805-9_14.
Oikawa T, Oyama M, Kozuka-Hata H, Uehara S, Udagawa N, Saya H, et al. Tks5-dependent formation of circumferential podosomes/invadopodia mediates cell-cell fusion. J Cell Biol. 2012;197:553–68. https://doi.org/10.1083/jcb.201111116.
Ono T, Nakashima T. Recent advances in osteoclast biology. Histochem Cell Biol. 2018;149:325–41. https://doi.org/10.1007/s00418-018-1636-2.
Matsumoto M, Kogawa M, Wada S, Takayanagi H, Tsujimoto M, Katayama S, et al. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J Biol Chem. 2004;279:45969–79. https://doi.org/10.1074/jbc.M408795200.
Zhou F, Shen Y, Liu B, Chen X, Wan L, Peng D. Gastrodin inhibits osteoclastogenesis via down-regulating the NFATc1 signaling pathway and stimulates osseointegration in vitro. Biochem Biophys Res Commun. 2017;484:820–6. https://doi.org/10.1016/j.bbrc.2017.01.179.
Sivakumar B, Akhavani MA, Winlove CP, Taylor PC, Paleolog EM, Kang N. Synovial hypoxia as a cause of tendon rupture in rheumatoid arthritis. J Hand Surg Am. 2008;33:49–58. https://doi.org/10.1016/j.jhsa.2007.09.002.
Lund-Olesen K. Oxygen tension in synovial fluids. Arthritis Rheum. 1970;13:769–76. https://doi.org/10.1002/art.1780130606.
Treuhaft PS, DJ MC. Synovial fluid pH, lactate, oxygen and carbon dioxide partial pressure in various joint diseases. Arthritis Rheum. 1971;14:475–84. https://doi.org/10.1002/art.1780140407.
Knowles HJ. Hypoxic regulation of osteoclast differentiation and bone resorption activity. Hypoxia Auckl. 2015;3:73–82. https://doi.org/10.2147/HP.S95960.
Arnett TR, Gibbons DC, Utting JC, Orriss IR, Hoebertz A, Rosendaal M, et al. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol. 2003;196:2–8. https://doi.org/10.1002/jcp.10321.
Utting JC, Flanagan AM, Brandao-Burch A, Orriss IR, Arnett TR. Hypoxia stimulates osteoclast formation from human peripheral blood. Cell Biochem Funct. 2010;28:374–80. https://doi.org/10.1002/cbf.1660.
Muzylak M, Price JS, Horton MA. Hypoxia induces giant osteoclast formation and extensive bone resorption in the cat. Calcif Tissue Int. 2006;79:301–9. https://doi.org/10.1007/s00223-006-0082-7.
Leger AJ, Altobelli A, Mosquea LM, Belanger AJ, Song A, Cheng SH, et al. Inhibition of osteoclastogenesis by prolyl hydroxylase inhibitor dimethyloxallyl glycine. J Bone Miner Metab. 2010;28:510–9. https://doi.org/10.1007/s00774-010-0171-6.
Kang H, Yang K, Xiao L, Guo L, Guo C, Yan Y, et al. Osteoblast hypoxia-inducible factor-1alpha pathway activation restrains osteoclastogenesis via the interleukin-33-MicroRNA-34a-Notch1 pathway. Front Immunol. 2017;8:1312. https://doi.org/10.3389/fimmu.2017.01312.
Hulley PA, Bishop T, Vernet A, Schneider JE, Edwards JR, Athanasou NA, et al. Hypoxia-inducible factor 1-alpha does not regulate osteoclastogenesis but enhances bone resorption activity via prolyl-4-hydroxylase 2. J Pathol. 2017;242:322–33. https://doi.org/10.1002/path.4906.
Yu R, Li C, Sun L, Jian L, Ma Z, Zhao J, et al. Hypoxia induces production of citrullinated proteins in human fibroblast-like synoviocytes through regulating HIF1alpha. Scand J Immunol. 2018;87:e12654. https://doi.org/10.1111/sji.12654.
Knowles HJ, Athanasou NA. Acute hypoxia and osteoclast activity: a balance between enhanced resorption and increased apoptosis. J Pathol. 2009;218:256–64. https://doi.org/10.1002/path.2534.
Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. https://doi.org/10.1038/nrc3064.
Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–64. https://doi.org/10.1038/nrc2501.
Shao J, Zhang Y, Yang T, Qi J, Zhang L, Deng L. HIF-1alpha disturbs osteoblasts and osteoclasts coupling in bone remodeling by up-regulating OPG expression. In Vitro Cell Dev Biol Anim. 2015;51:808–14. https://doi.org/10.1007/s11626-015-9895-x.
Saleh H, Eeles D, Hodge JM, Nicholson GC, Gu R, Pompolo S, et al. Interleukin-33, a target of parathyroid hormone and oncostatin m, increases osteoblastic matrix mineral deposition and inhibits osteoclast formation in vitro. Endocrinology. 2011;152:1911–22. https://doi.org/10.1210/en.2010-1268.
Schulze J, Bickert T, Beil FT, Zaiss MM, Albers J, Wintges K, et al. Interleukin-33 is expressed in differentiated osteoblasts and blocks osteoclast formation from bone marrow precursor cells. J Bone Miner Res. 2011;26:704–17. https://doi.org/10.1002/jbmr.269.
Wu C, Rankin EB, Castellini L, Alcudia JF, LaGory EL, Andersen R, et al. Oxygen-sensing PHDs regulate bone homeostasis through the modulation of osteoprotegerin. Genes Dev. 2015;29:817–31. https://doi.org/10.1101/gad.255.000.114.
Song F, Zhou L, Zhao J, Liu Q, Yang M, Tan R, et al. Eriodictyol inhibits RANKL-induced osteoclast formation and function via inhibition of NFATC1 activity. J Cell Physiol. 2016;231(9):1983–93. https://doi.org/10.1002/jcp.25304.
Quiñonez-Flores CM, González-Chávez SA, Pacheco-Tena C. Hypoxia and its implications in rheumatoid arthritis. J Biomed Sci. 2016;23(1):62. https://doi.org/10.1186/s12929-016-0281-0.
Acknowledgements
We gratefully thank the Medical Research Center of Peking University Third Hospital for kindly providing the RAW264.7 cells. We thank the Medical Research Center of Peking University Third Hospital for providing experimental equipment and technical support.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81471599) and the National Young Scholars Foundation of China (No. 81501387), the Prairie Fire Program (LYJH-92).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Additional information
Responsible Editor: Jason J. McDougall.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, Z., Yu, R., Zhao, J. et al. Constant hypoxia inhibits osteoclast differentiation and bone resorption by regulating phosphorylation of JNK and IκBα. Inflamm. Res. 68, 157–166 (2019). https://doi.org/10.1007/s00011-018-1209-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-018-1209-9