Abstract
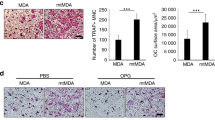
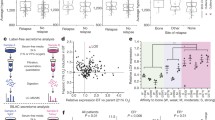
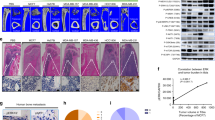
Since bone metastatic breast cancer is an incurable disease, causing significant morbidity and mortality, an understanding of the underlying molecular mechanisms would be highly valuable. Here, we describe in vitro and in vivo evidences for the importance of serine biosynthesis in the metastasis of breast cancer to bone. We first characterized the bone metastatic propensity of the MDA-MB-231(SA) cell line variant as compared to the parental MDA-MB-231 cells by radiographic and histological observations in the inoculated mice. Genome-wide gene expression profiling of this isogenic cell line pair revealed that all the three genes involved in the l-serine biosynthesis pathway, phosphoglycerate dehydrogenase (PHGDH), phosphoserine aminotransferase 1 (PSAT1), and phosphoserine phosphatase (PSPH) were upregulated in the highly metastatic variant. This pathway is the primary endogenous source for l-serine in mammalian tissues. Consistently, we observed that the proliferation of MDA-MB-231(SA) cells in serine-free conditions was dependent on PSAT1 expression. In addition, we observed that l-serine is essential for the formation of bone resorbing human osteoclasts and may thus contribute to the vicious cycle of osteolytic bone metastasis. High expression of PHGDH and PSAT1 in primary breast cancer was significantly associated with decreased relapse-free and overall survival of patients and malignant phenotypic features of breast cancer. In conclusion, high expression of serine biosynthesis genes in metastatic breast cancer cells and the stimulating effect of l-serine on osteoclastogenesis and cancer cell proliferation indicate a functionally critical role for serine biosynthesis in bone metastatic breast cancer and thereby an opportunity for targeted therapeutic interventions.






Similar content being viewed by others
References
Vargas SJ, Gillespie MT, Powell GJ et al (1992) Localization of parathyroid hormone-related protein mRNA expression in breast cancer and metastatic lesions by in situ hybridization. J Bone Miner Res 7:971–979
Guise TA, Yin JJ, Taylor SD et al (1996) Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Investig 98:1544–1549. doi:10.1172/JCI118947
Akhtari M, Mansuri J, Newman KA et al (2008) Biology of breast cancer bone metastasis. Cancer Biol Ther 7:3–9
Yin JJ, Selander K, Chirgwin JM et al (1999) TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Investig 103:197–206. doi:10.1172/JCI3523
Kang Y, Siegel PM, Shu W et al (2003) A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3:537–549
Yoneda T, Williams PJ, Hiraga T et al (2001) A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res 16:1486–1495
Bendre MS, Margulies AG, Walser B et al (2005) Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res 65:11001–11009. doi:10.1158/0008-5472.CAN-05-2630
Kakonen SM, Selander KS, Chirgwin JM et al (2002) Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. J Biol Chem 277:24571–24578. doi:10.1074/jbc.M202561200
R Development Core Team (2004) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria
Gentleman RC, Carey VJ, Bates DM et al (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5:R80. doi:10.1186/gb-2004-5-10-r80
Irizarry RA, Bolstad BM, Collin F et al (2003) Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31:e15
Dai M, Wang P, Boyd AD et al (2005) Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res 33:e175. doi:10.1093/nar/gni179
Miller LD, Smeds J, George J et al (2005) An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci USA 102:13550–13555. doi:10.1073/pnas.0506230102
Sorlie T, Tibshirani R, Parker J et al (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100:8418–8423. doi:10.1073/pnas.0932692100
Dennis G Jr, Sherman BT, Hosack DA et al (2003) DAVID: database for annotation, visualization, and integrated discovery. Genome Biol 4:P3
Zhang B, Kirov S, Snoddy J (2005) WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res 33:W741–W748. doi:10.1093/nar/gki475
Lewis RM, Glazier J, Greenwood SL et al (2007) l-serine uptake by human placental microvillous membrane vesicles. Placenta 28:445–452. doi:10.1016/j.placenta.2006.06.014
Ogawa T, Ishida-Kitagawa N, Tanaka A et al (2006) A novel role of l-serine (l-Ser) for the expression of nuclear factor of activated T cells (NFAT)2 in receptor activator of nuclear factor kappa B ligand (RANKL)-induced osteoclastogenesis in vitro. J Bone Miner Metab 24:373–379. doi:10.1007/s00774-006-0705-0
Alatalo SL, Halleen JM, Hentunen TA et al (2000) Rapid screening method for osteoclast differentiation in vitro that measures tartrate-resistant acid phosphatase 5b activity secreted into the culture medium. Clin Chem 46:1751–1754
Snell K (1984) Enzymes of serine metabolism in normal, developing and neoplastic rat tissues. Adv Enzyme Regul 22:325–400
Arriza JL, Kavanaugh MP, Fairman WA et al (1993) Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J Biol Chem 268:15329–15332
Broer A, Wagner C, Lang F et al (2000) Neutral amino acid transporter ASCT2 displays substrate-induced Na+ exchange and a substrate-gated anion conductance. Biochem J 346(Pt 3):705–710
Chaudhry FA, Schmitz D, Reimer RJ et al (2002) Glutamine uptake by neurons: interaction of protons with system a transporters. J Neurosci 22:62–72
Fukasawa Y, Segawa H, Kim JY et al (2000) Identification and characterization of a Na(+)-independent neutral amino acid transporter that associates with the 4F2 heavy chain and exhibits substrate selectivity for small neutral d- and l-amino acids. J Biol Chem 275:9690–9698
de Koning TJ, Snell K, Duran M et al (2003) l-serine in disease and development. Biochem J 371:653–661. doi:10.1042/BJ20021785
Martens JW, Nimmrich I, Koenig T et al (2005) Association of DNA methylation of phosphoserine aminotransferase with response to endocrine therapy in patients with recurrent breast cancer. Cancer Res 65:4101–4117. doi:10.1158/0008-5472.CAN-05-0064
Kozlow W, Guise TA (2005) Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia 10:169–180. doi:10.1007/s10911-005-5399-8
Acknowledgements
This study was supported by the Academy of Finland Center of Excellence grant (Center of Excellence in Translational Genome-Scale Biology 2006–2011), TIME (Disseminated Tumour Cells as Targets for Inhibiting Metastasis of Epithelial Tumours) project, Drug Discovery Graduate School, NIH grant R01 CA69158 from the National Cancer Institute, and by grants from the Finnish Cancer Organisations, the Sigrid Jusélius Foundation, and the Finnish Cultural Foundation. We thank Barry G. Grubbs and Rami Käkönen for excellent technical assistance in the mouse studies, Pharmatest Services Ltd (http://www.pharmatest.fi/) for technical help and discussions regarding the osteoclast cultures, and John Patrick Mpindi for help in bioinformatic analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10549-011-1654-4
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pollari, S., Käkönen, SM., Edgren, H. et al. Enhanced serine production by bone metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res Treat 125, 421–430 (2011). https://doi.org/10.1007/s10549-010-0848-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-0848-5