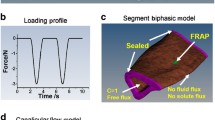
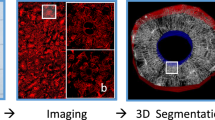
Abstract
Purpose of Review
Solute transport in the lacunar-canalicular system (LCS) plays important roles in osteocyte metabolism and cell-cell signaling. This review will summarize recent studies that establish pericellular matrix (PCM), discovered inside the LCS, as a crucial regulator of solute transport in bone.
Recent Findings
Utilizing confocal imaging and mathematical modeling, recent studies successfully quantified molecular diffusion and convection in the LCS as well as the size-dependent sieving effects of the PCM, leading to the quantification of the effective PCM fiber spacing (10 to 17 nm) in murine adult bones. Perlecan/HSPG2, a large linear proteoglycan, was identified to be an essential PCM component.
Summary
The PCM-filled LCS is bone’s chromatographic column, where fluid/solute transport to and from the osteocytes is regulated. The chemical composition, deposition rate, and turnover rate of the osteocyte PCM should be further defined to better understand osteocyte physiology and bone metabolism.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38. https://doi.org/10.1002/jbmr.320.
• Schaffler MB, et al. Osteocytes: master orchestrators of bone. Calcif Tissue Int. 2014;94(1):5–24. Provided a comprehensive review on osteocyte cell-cell signaling in repairing tissue damage and remodeling.
Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102(2):274–82. https://doi.org/10.1172/JCI2799.
Busse B, Djonic D, Milovanovic P, Hahn M, Püschel K, Ritchie RO, et al. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell. 2010;9(6):1065–75. https://doi.org/10.1111/j.1474-9726.2010.00633.x.
Qiu S, Rao DS, Palnitkar S, Parfitt AM. Reduced iliac cancellous osteocyte density in patients with osteoporotic vertebral fracture. J Bone Miner Res. 2003;18(9):1657–63. https://doi.org/10.1359/jbmr.2003.18.9.1657.
Piekarski K, Munro M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature. 1977;269(5623):80–2. https://doi.org/10.1038/269080a0.
Fritton SP, Weinbaum S. Fluid and solute transport in bone: flow-induced mechanotransduction. Annu Rev Fluid Mech. 2009;41(1):347–74. https://doi.org/10.1146/annurev.fluid.010908.165136.
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370(5):412–20. https://doi.org/10.1056/NEJMoa1305224.
Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell ... and more. Endocr Rev. 2013;34(5):658–90. https://doi.org/10.1210/er.2012-1026.
Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006;235(1):176–90. https://doi.org/10.1002/dvdy.20603.
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22(23):6267–76. https://doi.org/10.1093/emboj/cdg599.
Zhao S, Kato Y, Zhang Y, Harris S, Ahuja SS, Bonewald LF. MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J Bone Miner Res. 2002;17(11):2068–79. https://doi.org/10.1359/jbmr.2002.17.11.2068.
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17(10):1235–41. https://doi.org/10.1038/nm.2448.
You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, et al. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008;42(1):172–9. https://doi.org/10.1016/j.bone.2007.09.047.
Duan P, Bonewald LF. The role of the wnt/beta-catenin signaling pathway in formation and maintenance of bone and teeth. Int J Biochem Cell Biol. 2016;77(Pt A):23–9.
Riquelme MA, Burra S, Kar R, Lampe PD, Jiang JX. Mitogen-activated protein kinase (MAPK) activated by prostaglandin E2 phosphorylates connexin 43 and closes osteocytic hemichannels in response to continuous flow shear stress. J Biol Chem. 2015;290(47):28321–8. https://doi.org/10.1074/jbc.M115.683417.
de Castro LF, Maycas M, Bravo B, Esbrit P, Gortazar A. VEGF receptor 2 (VEGFR2) activation is essential for osteocyte survival induced by mechanotransduction. J Cell Physiol. 2015;230(2):278–85. https://doi.org/10.1002/jcp.24734.
Kitase Y, Barragan L, Qing H, Kondoh S, Jiang JX, Johnson ML, et al. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the beta-catenin and PKA pathways. J Bone Miner Res. 2010;25(12):2657–68. https://doi.org/10.1002/jbmr.168.
Zeng Y, Cowin SC, Weinbaum S. A fiber matrix model for fluid flow and streaming potentials in the canaliculi of an osteon. Ann Biomed Eng. 1994;22(3):280–92. https://doi.org/10.1007/BF02368235.
Burger EH, Klein-Nulend J. Mechanotransduction in bone—role of the lacuno-canalicular network. FASEB J. 1999;13(Suppl):S101–12.
Cardoso L, Fritton SP, Gailani G, Benalla M, Cowin SC. Advances in assessment of bone porosity, permeability and interstitial fluid flow. J Biomech. 2013;46(2):253–65. https://doi.org/10.1016/j.jbiomech.2012.10.025.
Cowin SC. Bone poroelasticity. J Biomech. 1999;32(3):217–38. https://doi.org/10.1016/S0021-9290(98)00161-4.
Dyke JP, Aaron RK. Noninvasive methods of measuring bone blood perfusion. Ann N Y Acad Sci. 2010;1192(1):95–102. https://doi.org/10.1111/j.1749-6632.2009.05376.x.
• Granke M, Does MD, Nyman JS. The role of water compartments in the material properties of cortical bone. Calcif Tissue Int. 2015;97(3):292–307. Provided a review on bound water and free water distribution in bone tissue and their roles in bone material properties.
Aarden EM, Burger EH, Nijweide PJ. Function of osteocytes in bone. J Cell Biochem. 1994;55(3):287–99. https://doi.org/10.1002/jcb.240550304.
Pienkowski D, Pollack SR. The origin of stress-generated potentials in fluid-saturated bone. J Orthop Res. 1983;1(1):30–41. https://doi.org/10.1002/jor.1100010105.
Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27(3):339–60. https://doi.org/10.1016/0021-9290(94)90010-8.
Cowin SC, Weinbaum S, Zeng Y. A case for bone canaliculi as the anatomical site of strain generated potentials. J Biomech. 1995;28(11):1281–97. https://doi.org/10.1016/0021-9290(95)00058-P.
You L, Cowin SC, Schaffler MB, Weinbaum S. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J Biomech. 2001;34(11):1375–86. https://doi.org/10.1016/S0021-9290(01)00107-5.
Wang Y, McNamara LM, Schaffler MB, Weinbaum S. A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc Natl Acad Sci U S A. 2007;104(40):15941–6. https://doi.org/10.1073/pnas.0707246104.
Han Y, Cowin SC, Schaffler MB, Weinbaum S. Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci U S A. 2004;101(47):16689–94. https://doi.org/10.1073/pnas.0407429101.
Webster DJ, Schneider P, Dallas SL, Müller R. Studying osteocytes within their environment. Bone. 2013;54(2):285–95. https://doi.org/10.1016/j.bone.2013.01.004.
Li W, You L, Schaffler MB, Wang L. The dependency of solute diffusion on molecular weight and shape in intact bone. Bone. 2009;45(5):1017–23. https://doi.org/10.1016/j.bone.2009.07.076.
Price C, Zhou X, Li W, Wang L. Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: direct evidence for load-induced fluid flow. J Bone Miner Res. 2011;26(2):277–85. https://doi.org/10.1002/jbmr.211.
•• Wang B, et al. Perlecan-containing pericellular matrix regulates solute transport and mechanosensing within the osteocyte lacunar-canalicular system. J Bone Miner Res. 2014;29(4):878–91. Quantified diffusion and convection in perlecan-deficient bone, measured reflection coefficient of PCM of normal and decreased perlecan expression, and determined the effective fiber spacing for the PCM. Linked cellular hydraulic forces (shear and drag) with in vivo bone formation and provided evidence that perlecan serves as a flow sensor in the LCS.
Wang L, Wang Y, Han Y, Henderson SC, Majeska RJ, Weinbaum S, et al. In situ measurement of solute transport in the bone lacunar-canalicular system. Proc Natl Acad Sci U S A. 2005;102(33):11911–6. https://doi.org/10.1073/pnas.0505193102.
Wang L, Ciani C, Doty SB, Fritton SP. Delineating bone’s interstitial fluid pathway in vivo. Bone. 2004;34(3):499–509. https://doi.org/10.1016/j.bone.2003.11.022.
Tami AE, Schaffler MB, Knothe Tate ML. Probing the tissue to subcellular level structure underlying bone’s molecular sieving function. Biorheology. 2003;40(6):577–90.
• Ciani C, et al. Ovariectomy enhances mechanical load-induced solute transport around osteocytes in rat cancellous bone. Bone. 2014;59:229–34. Provided evidence that estrogen deficiency altered solute perfusion in cortical and trabecular bone.
Lang SB, Stipanich N, Soremi EA. Diffusion of glucose in stressed and unstressed canine femur in vitro. Ann N Y Acad Sci. 1974;238(1 Electrically):139–48. https://doi.org/10.1111/j.1749-6632.1974.tb26784.x.
Fernandez-Seara MA, Wehrli SL, Wehrli FW. Diffusion of exchangeable water in cortical bone studied by nuclear magnetic resonance. Biophys J. 2002;82(1 Pt 1):522–9. https://doi.org/10.1016/S0006-3495(02)75417-9.
Li W, Gardinier JD, Price C, Wang L. Does blood pressure enhance solute transport in the bone lacunar-canalicular system? Bone. 2010;47(2):353–9. https://doi.org/10.1016/j.bone.2010.05.005.
•• Wang B, et al. Quantifying load-induced solute transport and solute-matrix interaction within the osteocyte lacunar-canalicular system. J Bone Miner Res. 2013;28(5):1075–86. Developed the FRAP-based velocimetry to quantify reflection coefficient of the PCM and the molecular sieving model to derive the effective fiber spacing.
Knothe Tate ML, Knothe U. An ex vivo model to study transport processes and fluid flow in loaded bone. J Biomech. 2000;33(2):247–54. https://doi.org/10.1016/S0021-9290(99)00143-8.
Zhou X, Novotny JE, Wang L. Modeling fluorescence recovery after photobleaching in loaded bone: potential applications in measuring fluid and solute transport in the osteocytic lacunar-canalicular system. Ann Biomed Eng. 2008;36(12):1961–77. https://doi.org/10.1007/s10439-008-9566-0.
Hillsley MV, Frangos JA. Bone tissue engineering: the role of interstitial fluid flow. Biotechnol Bioeng. 1994;43(7):573–81. https://doi.org/10.1002/bit.260430706.
You LD, Weinbaum S, Cowin SC, Schaffler MB. Ultrastructure of the osteocyte process and its pericellular matrix. Anat Rec A Discov Mol Cell Evol Biol. 2004;278(2):505–13. https://doi.org/10.1002/ar.a.20050.
Thompson WR, Modla S, Grindel BJ, Czymmek KJ, Kirn-Safran CB, Wang L, et al. Perlecan/Hspg2 deficiency alters the pericellular space of the lacunocanalicular system surrounding osteocytic processes in cortical bone. J Bone Miner Res. 2011;26(3):618–29. https://doi.org/10.1002/jbmr.236.
Iozzo RV, Cohen IR, Grässel S, Murdoch AD. The biology of perlecan: the multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem J. 1994;302(Pt 3):625–39. https://doi.org/10.1042/bj3020625.
• Farach-Carson MC, et al. Border patrol: insights into the unique role of perlecan/heparan sulfate proteoglycan 2 at cell and tissue borders. Matrix Biol. 2014;34:64–79. Provided a comprehensive review on perlecan’s structural and biological functions along various tissue interfaces including bone LCS.
•• Wijeratne SS, et al. Single molecule force measurements of perlecan/HSPG2: a key component of the osteocyte pericellular matrix. Matrix Biol. 2016;50:27–38. AFM imaging of the dimensions of isolated human perlecan and tested the mechanical strength of the perlecan core proteins by AFM pulling experiments.
Rodgers KD, Sasaki T, Aszodi A, Jacenko O. Reduced perlecan in mice results in chondrodysplasia resembling Schwartz-Jampel syndrome. Hum Mol Genet. 2007;16(5):515–28. https://doi.org/10.1093/hmg/ddl484.
• Lai X et al. The dependences of osteocyte network on bone compartment, age, and disease. Bone Res, 2015. 3. Investigated the variations of the LCS in murine bone and discovered the relatively constant of the canalicular number density per unit lacunar surface.
Lynch ME, Main RP, Xu Q, Schmicker TL, Schaffler MB, Wright TM, et al. Tibial compression is anabolic in the adult mouse skeleton despite reduced responsiveness with aging. Bone. 2011;49(3):439–46. https://doi.org/10.1016/j.bone.2011.05.017.
• Fan L, et al. A multiscale 3D finite element analysis of fluid/solute transport in mechanically loaded bone. Bone Res. 2016;4:16032. Modeling fluid and solute transport at three levels (whole bone, lacunar, and canalicular levels).
Martinez, J.R., S. Pei, and L. Wang. Profiling the composition of osteocyte pericellular matrix (PCM) in vivo and in vitro. in Annual meeting of American Association for Bone and Mineral Research. 2017. Denver, CO.
Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42(6):551–5. https://doi.org/10.1016/j.injury.2011.03.031.
Topping J, Black AJ, Farquharson RG, Fraser WD. Osteoporosis in pregnancy: more than postural backache. Prof Care Mother Child. 1998;8(6):147–50.
•• Jahn K, et al. Osteocytes acidify their microenvironment in response to PTHrP in vitro and in lactating mice in vivo. J Bone Miner Res. 2017;32(8):1761–72. New evidence that osteocytes are capable of remodeling the surrounding matrix.
•• Nango N, et al. Osteocyte-directed bone demineralization along canaliculi. Bone. 2016;84:279–88. New evidence that osteocytes are capable of remodeling the surrounding matrix.
Qing H, Ardeshirpour L, Divieti Pajevic P, Dusevich V, Jähn K, Kato S, et al. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012;27(5):1018–29. https://doi.org/10.1002/jbmr.1567.
Dallas SL, Bonewald LF. Dynamics of the transition from osteoblast to osteocyte. Ann N Y Acad Sci. 2010;1192(1):437–43. https://doi.org/10.1111/j.1749-6632.2009.05246.x.
•• Kamioka H, et al. Microscale fluid flow analysis in a human osteocyte canaliculus using a realistic high-resolution image-based three-dimensional model. Integr Biol (Camb). 2012;4(10):1198–206. Irregular canalicular wall and rough surface were observed and osteocyte cell processes appeared not in direct contact with canalicular wall under 2.2 nm resolution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Liyun Wang received grant support from the National Institutes of Health (R01AR054385) and declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Osteocytes
Rights and permissions
About this article
Cite this article
Wang, L. Solute Transport in the Bone Lacunar-Canalicular System (LCS). Curr Osteoporos Rep 16, 32–41 (2018). https://doi.org/10.1007/s11914-018-0414-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-018-0414-3