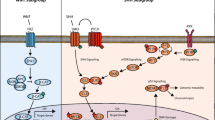
Abstract
Medulloblastoma is a malignant embryonic brain tumor arising in the posterior fossa and typically occurring in pediatric patients. Current multimodal treatment regimes have significantly improved the survival rates; however, a marked heterogeneity in therapy response is observed, and one third of all patients die within 5 years after diagnosis. Large-scale genetic and transcriptome analysis revealed four medulloblastoma subgroups (WNT, SHH, Group 3, and Group 4) associated with different demographic parameters, tumor manifestation, and clinical behavior. Future treatment protocols will integrate molecular classification schemes to evaluate subgroup-specific intensification or de-escalation of adjuvant therapies aimed to increase tumor control and reduce iatrogenic induced morbidity. Furthermore, the identification of genetic drivers allows assessing target therapies in order to increase the chemotherapeutic armamentarium. This review highlights the biology behind the current classification system and elucidates relevant aspects of the disease influencing forthcoming clinical trials.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14 Suppl 5:1–49.
Smoll NR, Drummond KJ. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci. 2012;19(11):1541–4.
Park TS, Hoffman HJ, Hendrick EB, et al. Medulloblastoma: clinical presentation and management. Experience at the hospital for sick children, toronto, 1950–1980. J Neurosurg. 1983;58(4):543–52.
Sutton LN, Phillips PC, Molloy PT. Surgical management of medulloblastoma. J Neurooncol. 1996;29(1):9–21.
Northcott PA, Korshunov A, Witt H, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2010;29(11):1408–14. Northcott et al. identified four different subgroups in a large cohort of medulloblastomas with disparate demographics, clinical presentation, transcriptional profiles, genetic abnormalities, and clinical outcome by an integrative genomic approach.
Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465–72. In a consensus conference in Boston held in 2010, experts from diferent laboratories agreed that the evidence supported the existence of four main subgroups of medulloblastoma as Wnt, Shh, Group 3, and Group 4. Participants outlined the demographic, transcriptional, genetic, and clinical differences between the four subgroups.
Faria CC, Golbourn BJ, Dubuc AM, et al. Foretinib is effective therapy for metastatic sonic hedgehog medulloblastoma. Cancer Res. 2015;75(1):134–46.
Gibson P, Tong Y, Robinson G, et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468(7327):1095–9. The authors first reported data supporting that subtypes of medulloblastoma have distinct cellular origins. Their results also showed prominent molecular and clinical differences between SHH- and WNT-subtype medulloblastomas.
Bailey PCH. Medulloblastoma cerebelli. Arch Neurol Psychiatry. 1925;14:192–223.
Rutka JT, Hoffman HJ. Medulloblastoma: a historical perspective and overview. J Neurooncol. 1996;29(1):1–7.
Pomeroy SL, Tamayo P, Gaasenbeek M, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415(6870):436–42.
Gilbertson RJ, Ellison DW. The origins of medulloblastoma subtypes. Annu Rev Pathol. 2008;3:341–65.
Klein C, Butt SJ, Machold RP, et al. Cerebellum- and forebrain-derived stem cells possess intrinsic regional character. Development. 2005;132(20):4497–508.
Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13(12):767–79.
Villavicencio EH, Walterhouse DO, Iannaccone PM. The sonic hedgehog-patched-Gli pathway in human development and disease. Am J Hum Genet. 2000;67(5):1047–54.
Shahi MH, Rey JA, Castresana JS. The sonic hedgehog-GLI1 signaling pathway in brain tumor development. Expert Opin Ther Targets. 2012;16(12):1227–38.
Varjosalo M, Taipale J. Hedgehog signaling. J Cell Sci. 2007;120(Pt 1):3–6.
Lee Y, Kawagoe R, Sasai K, et al. Loss of suppressor-of-fused function promotes tumorigenesis. Oncogene. 2007;26(44):6442–7.
Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci. 2005;6(5):351–62.
Li VS, Ng SS, Boersema PJ, et al. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell. 2012;149(6):1245–56.
Northcott PA, Jones DT, Kool M, et al. Medulloblastomics: the end of the beginning. Nat Rev Cancer. 2012;12(12):818–34.
Danysh HE, Mitchell LE, Zhang K, et al. Traffic-related air pollution and the incidence of childhood central nervous system tumors: Texas, 2001–2009. Pediatr Blood Cancer. 2015;62(9):1572–8.
Bonaventure A, Simpson J, Ansell P, et al. Prescription drug use during pregnancy and risk of childhood cancer - is there an association? Cancer Epidemiol. 2015;39(1):73–8.
Hawkins C, Croul S. Viruses and human brain tumors: cytomegalovirus enters the fray. J Clin Invest. 2011;121(10):3831–3.
Evans G, Burnell L, Campbell R, et al. Congenital anomalies and genetic syndromes in 173 cases of medulloblastoma. Med Pediatr Oncol. 1993;21(6):433–4.
Gajjar AJ, Robinson GW. Medulloblastoma-translating discoveries from the bench to the bedside. Nat Rev Clin Oncol. 2014;11(12):714–22.
Smith MJ, Beetz C, Williams SG, et al. Germline mutations in SUFU cause Gorlin syndrome-associated childhood medulloblastoma and redefine the risk associated with PTCH1 mutations. J Clin Oncol. 2014;32(36):4155–61.
Hamilton SR, Liu B, Parsons RE, et al. The molecular basis of Turcot’s syndrome. N Engl J Med. 1995;332(13):839–47.
Rausch T, Jones DT, Zapatka M, et al. Genome sequencing of pediatric medulloblastoma links catastrophic DNA rearrangements with TP53 mutations. Cell. 2012;148(1–2):59–71.
Kool M, Korshunov A, Remke M, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123(4):473–84. This meta-analysis assessed molecular and clinical data of medulloblastomas brought together from seven independent studies, which showed mostly congruent results of molecular subtypes with respect to transcriptome profile, DNA copy-number aberrations, demographics, and survival.
Katsetos CD, Liu HM, Zacks SI. Immunohistochemical and ultrastructural observations on Homer Wright (neuroblastic) rosettes and the “pale islands” of human cerebellar medulloblastomas. Hum Pathol. 1988;19(10):1219–27.
Ellison D. Classifying the medulloblastoma: insights from morphology and molecular genetics. Neuropathol Appl Neurobiol. 2002;28(4):257–82.
Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109.
Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–20.
Leary SE, Zhou T, Holmes E, et al. Histology predicts a favorable outcome in young children with desmoplastic medulloblastoma: a report from the children’s oncology group. Cancer. 2011;117(14):3262–7.
Grill J, Sainte-Rose C, Jouvet A, et al. Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol. 2005;6(8):573–80.
Tarbell NJ, Friedman H, Polkinghorn WR, et al. High-risk medulloblastoma: a pediatric oncology group randomized trial of chemotherapy before or after radiation therapy (POG 9031). J Clin Oncol. 2013;31(23):2936–41.
Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352(10):978–86.
Duffner PK, Horowitz ME, Krischer JP, et al. The treatment of malignant brain tumors in infants and very young children: an update of the Pediatric Oncology Group experience. Neuro Oncol. 1999;1(2):152–61.
Uday S, Murray RD, Picton S, et al. Endocrine sequelae beyond 10 years in survivors of medulloblastoma. Clin Endocrinol (Oxf). 2015;83(5):663–70.
Camara-Costa H, Resch A, Kieffer V, et al. Neuropsychological Outcome of Children Treated for Standard Risk Medulloblastoma in the PNET4 European Randomized Controlled Trial of Hyperfractionated Versus Standard Radiation Therapy and Maintenance Chemotherapy. Int J Radiat Oncol Biol Phys. 2015;92(5):978–85.
Packer RJ, Zhou T, Holmes E, et al. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children’s Oncology Group trial A9961. Neuro Oncol. 2013;15(1):97–103.
Ning MS, Perkins SM, Dewees T, et al. Evidence of high mortality in long term survivors of childhood medulloblastoma. J Neurooncol. 2015;122(2):321–7.
Chang CH, Housepian EM, Herbert Jr C. An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93(6):1351–9.
Packer RJ, Vezina G. Management of and prognosis with medulloblastoma: therapy at a crossroads. Arch Neurol. 2008;65(11):1419–24.
Packer RJ, Goldwein J, Nicholson HS, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: a Children’s Cancer Group Study. J Clin Oncol. 1999;17(7):2127–36.
Louis DN, Perry A, Burger P, et al. International Society Of Neuropathology--Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014;24(5):429–35.
Thompson MC, Fuller C, Hogg TL, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24(12):1924–31.
Northcott PA, Shih DJ, Peacock J, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488(7409):49–56. This study reported a wide range of somatic copy number aberrations assigned to the four molecular variants which help to identify relevant targets for subgroup specific therapy.
Shih DJ, Northcott PA, Remke M, et al. Cytogenetic prognostication within medulloblastoma subgroups. J Clin Oncol. 2014;32(9):886–96. The authors identified a panel of cytogenetic biomarkers across medulloblastoma subtypes, enabeling to assing patients into very high-risk and very low-risk groups. This study proposes combining subgroup and molecular biomarkers with clinical biomarkers to improve patient prognostication.
Pietsch T, Schmidt R, Remke M, et al. Prognostic significance of clinical, histopathological, and molecular characteristics of medulloblastomas in the prospective HIT2000 multicenter clinical trial cohort. Acta Neuropathol. 2014;128(1):137–49. The purpose of the study was to prospectively evaluate clinical, histopathological and molecular variables for outcome prediction in medulloblastoma patients. A simple clinico-pathological risk score was identified.
Northcott PA, Hielscher T, Dubuc A, et al. Pediatric and adult sonic hedgehog medulloblastomas are clinically and molecularly distinct. Acta Neuropathol. 2011;122(2):231–40.
Kool M, Jones DT, Jager N, et al. Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell. 2014;25(3):393–405.
Wefers AK, Warmuth-Metz M, Poschl J, et al. Subgroup-specific localization of human medulloblastoma based on pre-operative MRI. Acta Neuropathol. 2014;127(6):931–3.
Ohli J, Neumann JE, Grammel D, et al. Localization of SHH medulloblastoma in mice depends on the age at its initiation. Acta Neuropathol. 2015;130(2):307–9.
Zhukova N, Ramaswamy V, Remke M, et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol. 2013;31(23):2927–35. Zhukova et al. investigated the prognostic impact of TP53 mutations in pediatric medulloblastoma and found that TP53 mutations are enriched among SHH medulloblastomas, in which they indicate poor outcome and treatment failure in these patients.
Remke M, Ramaswamy V, Peacock J, et al. TERT promoter mutations are highly recurrent in SHH subgroup medulloblastoma. Acta Neuropathol. 2013;126(6):917–29.
Gajjar A, Pfister SM, Taylor MD, et al. Molecular insights into pediatric brain tumors have the potential to transform therapy. Clin Cancer Res. 2014;20(22):5630–40.
Northcott PA, Lee C, Zichner T, et al. Enhancer hijacking activates GFI1 family oncogenes in medulloblastoma. Nature. 2014;511(7510):428–34. In this study a series of prevalent, highly different genomic structural variants of groups 3 and 4, resulting in specific and mutually exclusive activation of proto-oncogenes was described. GFI1 and GFI1B are shown to be activated by ‘enhancer hijacking’ and identified as prominent medulloblastoma oncogenes.
Pfaff E, Remke M, Sturm D, et al. TP53 mutation is frequently associated with CTNNB1 mutation or MYCN amplification and is compatible with long-term survival in medulloblastoma. J Clin Oncol. 2010;28(35):5188–96.
Korshunov A, Remke M, Kool M, et al. Biological and clinical heterogeneity of MYCN-amplified medulloblastoma. Acta Neuropathol. 2012;123(4):515–27.
Rutkowski S, von Hoff K, Emser A, et al. Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J Clin Oncol. 2010;28(33):4961–8.
Riffaud L, Saikali S, Leray E, et al. Survival and prognostic factors in a series of adults with medulloblastomas. J Neurosurg. 2009;111(3):478–87.
Rochkind S, Blatt I, Sadeh M, et al. Extracranial metastases of medulloblastoma in adults: literature review. J Neurol Neurosurg Psychiatry. 1991;54(1):80–6.
Wang X, Dubuc AM, Ramaswamy V, et al. Medulloblastoma subgroups remain stable across primary and metastatic compartments. Acta Neuropathol. 2015;129(3):449–57. This study represents the largest primary-metastatic paired cohort used to analyse subgroup-specific molecular aberrations within the metastatic compartment. The acquired data supports the hypothesis that medulloblastoma subgroups arise from distinct cells of origin.
Mumert M, Dubuc A, Wu X, et al. Functional genomics identifies drivers of medulloblastoma dissemination. Cancer Res. 2012;72(19):4944–53.
Jenkins NC, Kalra RR, Dubuc A, et al. Genetic drivers of metastatic dissemination in sonic hedgehog medulloblastoma. Acta Neuropathol Commun. 2014;2:85.
Snuderl M, Batista A, Kirkpatrick ND, et al. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell. 2013;152(5):1065–76.
Ramaswamy V, Remke M, Bouffet E, et al. Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol. 2013;14(12):1200–7. This study determines subgroup-specific differences in medulloblastoma recurrence patterns and potential treatment strategies. It reveals that medulloblastomas do not switch subgroup at the time of recurrence and shows significant differences in timing and location of recurrence.
Poschl J, Koch A, Schuller U. Histological subtype of medulloblastoma frequently changes upon recurrence. Acta Neuropathol. 2015;129(3):459–61.
Clifford SC, Lannering B, Schwalbe EC, et al.: Biomarker-driven stratification of disease-risk in non-metastatic medulloblastoma: results from the multi-center HIT-SIOP-PNET4 clinical trial. Oncotarget. 2015. This paper shows that biomarkers validated across medulloblastomas groups and determined as high-risk prognosticators behave differently in clinically-defined standard-risk medulloblastoma.
Kocakaya S, Beier CP, Beier D. Chemotherapy increases long-term survival in patients with adult medulloblastoma-a literature-based meta-analysis. Neuro Oncol. 2016;18(3):408–16. doi:10.1093/neuonc/nov185.
Rutkowski S, Cohen B, Finlay J, et al. Medulloblastoma in young children. Pediatr Blood Cancer. 2010;54(4):635–7.
Michiels EM, Schouten-Van Meeteren AY, Doz F, et al. Chemotherapy for children with medulloblastoma. Cochrane Database Syst Rev. 2015;1:CD006678.
Goschzik T, Zur Muhlen A, Kristiansen G, et al. Molecular stratification of medulloblastoma: comparison of histological and genetic methods to detect Wnt activated tumours. Neuropathol Appl Neurobiol. 2015;41(2):135–44.
Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13(7):513–32.
Cimmino F, Scoppettuolo MN, Carotenuto M, et al. Norcantharidin impairs medulloblastoma growth by inhibition of Wnt/beta-catenin signaling. J Neurooncol. 2012;106(1):59–70.
Zinke J, Schneider FT, Harter PN, et al. beta-Catenin-Gli1 interaction regulates proliferation and tumor growth in medulloblastoma. Mol Cancer. 2015;14:17.
Salaroli R, Ronchi A, Buttarelli FR, et al. Wnt activation affects proliferation, invasiveness and radiosensitivity in medulloblastoma. J Neurooncol. 2015;121(1):119–27.
Migden MR, Guminski A, Gutzmer R, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16(6):716–28.
Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366(23):2171–9.
Gajjar A, Stewart CF, Ellison DW, et al. Phase I study of vismodegib in children with recurrent or refractory medulloblastoma: a pediatric brain tumor consortium study. Clin Cancer Res. 2013;19(22):6305–12.
Robinson GW, Orr BA, Wu G, et al. Vismodegib exerts targeted efficacy against recurrent sonic hedgehog-subgroup medulloblastoma: results from phase II pediatric brain tumor consortium studies PBTC-025B and PBTC-032. J Clin Oncol. 2015;33(24):2646–54.
Rudin CM, Hann CL, Laterra J, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361(12):1173–8.
Yauch RL, Dijkgraaf GJ, Alicke B, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326(5952):572–4.
Buonamici S, Williams J, Morrissey M, et al.: Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci Transl Med. 2010;2(51):51ra70.
Zhao X, Ponomaryov T, Ornell KJ, et al. RAS/MAPK Activation Drives Resistance to Smo Inhibition, Metastasis, and Tumor Evolution in Shh Pathway-Dependent Tumors. Cancer Res. 2015;75(17):3623–35.
Kim J, Aftab BT, Tang JY, et al. Itraconazole and arsenic trioxide inhibit Hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23(1):23–34.
Tang Y, Gholamin S, Schubert S, et al. Epigenetic targeting of Hedgehog pathway transcriptional output through BET bromodomain inhibition. Nat Med. 2014;20(7):732–40.
Ransohoff KJ, Sarin KY, Tang JY. Smoothened Inhibitors in Sonic Hedgehog Subgroup Medulloblastoma. J Clin Oncol. 2015;33(24):2692–4.
Lee MJ, Hatton BA, Villavicencio EH, et al. Hedgehog pathway inhibitor saridegib (IPI-926) increases lifespan in a mouse medulloblastoma model. Proc Natl Acad Sci U S A. 2012;109(20):7859–64.
Morfouace M, Shelat A, Jacus M, et al. Pemetrexed and gemcitabine as combination therapy for the treatment of Group3 medulloblastoma. Cancer Cell. 2014;25(4):516–29.
Bandopadhayay P, Bergthold G, London WB, et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer. 2014;61(7):1173–9.
A clinical and molecular risk-directed therapy for newly diagnosed medulloblastoma. https://clinicaltrials.gov/ct2/show/NCT01878617 Accessed October 2015. The aim of this trial is to stratify medulloblastoma treatment in a phase II clinical trial based on both clinical risk and molecular subgroups.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Daniel Coluccia, Carlyn Figuereido, Semra Isik, Christian Smith, and James T. Rutka declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Neuro-Oncology
Rights and permissions
About this article
Cite this article
Coluccia, D., Figuereido, C., Isik, S. et al. Medulloblastoma: Tumor Biology and Relevance to Treatment and Prognosis Paradigm. Curr Neurol Neurosci Rep 16, 43 (2016). https://doi.org/10.1007/s11910-016-0644-7
Published:
DOI: https://doi.org/10.1007/s11910-016-0644-7