Abstract
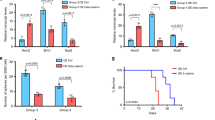
Medulloblastomas (MBs) associated with the Wnt activation represent a subgroup with a favorable prognosis, but it remains unclear whether Wnt activation confers a less aggressive phenotype and/or enhances radiosensitivity. To investigate this issue, we evaluated the biological behavior of an MB cell line, UW228-1, stably transfected with human β-catenin cDNA encoding a nondegradable form of β-catenin (UW-B) in standard culture conditions and after radiation treatment. We evaluated the expression, transcriptional activity, and localization of β-catenin in the stably transfected cells using immunofluorescence and WB. We performed morphological analysis using light and electron microscopy. We then analyzed changes in the invasiveness, growth, and mortality in standard culture conditions and after radiation. We demonstrated that (A) Wnt activation inhibited 97 % of the invasion capability of the cells, (B) the growth of the UW-B cells was statistically significantly lower than that of all the other control cells (p < 0.01), (C) the mortality of irradiated UW-B cells was statistically significantly higher than that of the controls and their nonirradiated counterparts (p < 0.05), and (D) morphological features of neuronal differentiation were observed in the Wnt-activated cells. In tissue samples, the Ki-67 labeling index (LI) was lower in β-catenin-positive samples compared to non-β-catenin positive ones. The Ki-67 LI median (LI = 40) of the nuclear β-catenin-positive tumor samples was lower than that of non-nuclear β-catenin-positive samples (LI = 50), but the difference was not statistically significant. Overall, our data suggest that activation of the Wnt pathway reduces the proliferation and invasion of MBs and increases the tumor’s radiosensitivity.









Similar content being viewed by others
References
Giangaspero F, Eberhart CG, Haapasalo H, Pietsch T, Wiestler OD, Ellison DW (2007) Medulloblastoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavanee WK (eds) WHO classification of tumors of the central nervous system. IARC, Lyon, pp 132–140
Gilbertson RJ, Ellison DW (2008) The origins of medulloblastoma subtypes. Annu Rev Pathol 3:341–365
Gessi M, von Bueren AO, Rutkowski S, Pietsch T (2012) p53 expression predicts dismal outcome for medulloblastoma patients with metastatic disease. J Neurooncol 106:135–140
Gilbertson RJ (2004) Medulloblastoma: signalling a change in treatment. Lancet Oncol 5:209–218
Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, Rutka JT, Pfister S, Taylor MD (2011) Medulloblastoma comprises four distinct molecular variants. J Clin Oncol 29:1408–1414
Pfister SM, Korshunov A, Kool M, Hasselblatt M, Eberhart C, Taylor MD (2010) Molecular diagnostics of CNS embryonal tumors. Acta Neuropathol 120:553–566
Eberhart CG (2011) Molecular diagnostics in embryonal brain tumors. Brain Pathol 21:96–104
Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, Kranenburg TA, Hogg T, Poppleton H, Martin J, Finkelstein D, Pounds S, Weiss A, Patay Z, Scoggins M, Ogg R, Pei Y, Yang ZJ, Brun S, Lee Y, Zindy F, Lindsey JC, Taketo MM, Boop FA, Sanford RA, Gajjar A, Clifford SC, Roussel MF, McKinnon PJ, Gutmann DH, Ellison DW, Wechsler-Reya R, Gilbertson RJ (2010) Subtypes of medulloblastoma have distinct developmental origins. Nature 468:1095–1099
Ellison DW, Dalton J, Kocak M, Nicholson SL, Fraga C, Neale G, Kenney AM, Brat DJ, Perry A, Yong WH, Taylor RE, Bailey S, Clifford SC, Gilbertson RJ (2011) Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol 121:381–396
Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, Cho YJ, Koster J, Schouten-van Meeteren A, van Vuurden D, Clifford SC, Pietsch T, von Bueren AO, Rutkowski S, McCabe M, Collins VP, Bäcklund ML, Haberler C, Bourdeaut F, Delattre O, Doz F, Ellison DW, Gilbertson RJ, Pomeroy SL, Taylor MD, Lichter P, Pfister SM (2012) Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol 123:473–484
Ellison DW, Onilude OE, Lindsey JC, Lusher ME, Weston CL, Taylor RE, Pearson AD, Clifford SC, United Kingdom Children’s Cancer Study Group Brain Tumour Committee (2005) Beta-catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom Children’s Cancer Study Group Brain Tumor Committee. J Clin Oncol 23:7951–7957
Fattet S, Haberler C, Legoix P, Varlet P, Lellouch-Tubiana A, Lair S, Manie E, Raquin MA, Bours D, Carpentier S, Barillot E, Grill J, Doz F, Puget S, Janoueix-Lerosey I, Delattre O (2009) Beta-catenin status in pediatric medulloblastomas: correlation of immunohistochemical expression with mutational status, genetic profiles, and clinical characteristics. J Pathol 218:86–94
Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, Chintagumpala M, Adesina A, Ashley DM, Kellie SJ, Taylor MD, Curran T, Gajjar A, Gilbertson RJ (2006) Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol 24:1924–1931
Cimmino F, Scoppettuolo MN, Carotenuto M, De Antonellis P, Dato VD, De Vita G, Zollo M (2012) Norcantharidin impairs medulloblastoma growth by inhibition of Wnt/b-catenin signaling. J Neurooncol 106:59–70
Clifford SC, Lusher ME, Lindsey JC, Langdon JA, Gilbertson RJ, Straughton D, Ellison DW (2006) Wnt/Wingless pathway activation and chromosome 6 loss characterize a distinct molecular sub-group of medulloblastomas associated with a favorable prognosis. Cell Cycle 5:2666–2670
Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P, Troost D, Meeteren NS, Caron HN, Cloos J, Mrsić A, Ylstra B, Grajkowska W, Hartmann W, Pietsch T, Ellison D, Clifford SC, Versteeg R (2008) Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One 3:e3088
Rogers HA, Miller S, Lowe J, Brundler MA, Coyle B, Grundy RG (2009) An investigation of WNT pathway activation and association with survival in central nervous system primitive neuroectodermal tumours (CNS PNET). Br J Cancer 100:1292–1302
Keles GE, Berger MS, Srinivasan J, Kolstoe DD, Bobola MS, Silber JR (1995) Establishment and characterization of four human medulloblastoma-derived cell lines. Oncol Res 7:493–503
Derksen PW, Tjin E, Meijer HP, Klok MD, MacGillavry HD, van Oers MH, Lokhorst HM, Bloem AC, Clevers H, Nusse R, van der Neut R, Spaargaren M, Pals ST (2004) Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci USA 101:6122–6127
Salaroli R, Di Tomaso T, Ronchi A, Ceccarelli C, Cammelli S, Cappellini A, Martinelli GN, Barbieri E, Giangaspero F, Cenacchi G (2008) Radiobiologic response of medulloblastoma cell lines: involvement of beta-catenin? J Neurooncol 90:243–251
Albini A, Iwamoto Y, Kleinman HK et al (1987) A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 47:3239–3245
Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, Woo S, Wheeler G, Ahern V, Krasin MJ, Fouladi M, Broniscer A, Krance R, Hale GA, Stewart CF, Dauser R, Sanford RA, Fuller C, Lau C, Boyett JM, Wallace D, Gilbertson RJ (2006) Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol 7:813–820
Peifer M, Polakis P (2000) Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science 2000:1606–1609
Polakis P (2000) Wnt signaling and cancer. Genes Dev 14:1837–1851
Clevers H (2006) Wnt/beta-catenin signalling in development and disease. Cell 127:469–480
Nager M, Bhardwaj D, Cantì C, Medina L, Nogués P, Herreros J (2012) β-catenin signalling in glioblastoma multiforme and glioma-initiating cells. Chemother Res Pract 2012:192362
Momota H, Shih AH, Edgar MA, Holland EC (2008) c-Myc and beta-catenin cooperate with loss of p53 to generate multiple members of the primitive neuroectodermal tumor family in mice. Oncogene 27:4392–4401
Zhao X, Liu Z, Yu L, Zhang Y, Baxter P, Voicu H, Gurusiddappa S, Luan J, Su JM, Leung HC, Li XN (2012) Global gene expression profiling confirms the molecular fidelity of primary tumor-based orthotopic xenograft mouse models of medulloblastoma. Neuro Oncol 14:574–583
Lucero OM, Dawson DW, Moon RT, Chien AJ (2010) A re-evaluation of the “oncogenic” nature of Wnt/beta-catenin signaling in melanoma and other cancers. Curr Oncol Rep 12:314–318
Pöschl J, Grammel D, Dorostkar MM, Batash R, Pöschl J, Schaffer PM (2013) Constitutive activation of beta-catenin in neural progenitors results in disrupted proliferation and migration of neurons within the central nervous system. Dev Biol 374:319–332
Acknowledgments
We thank Dr. Mike Bobola and Dr. Charles G. Eberhart for providing the human MB cell line UW228-1 and Dr. Hans Clevers for providing the plasmid construct.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salaroli, R., Ronchi, A., Buttarelli, F.R. et al. Wnt activation affects proliferation, invasiveness and radiosensitivity in medulloblastoma. J Neurooncol 121, 119–127 (2015). https://doi.org/10.1007/s11060-014-1621-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1621-0