Abstract
Infections of the human nervous system have substantial morbidity and mortality but also represent among the most challenging of all neurological diseases because of the difficulty in establishing a diagnosis and implementing effective therapies. Neurological infections lead to altered expression levels of a wide range of host- and pathogen-derived biomolecules both within and outside of the nervous system. Quantitative analyses of these biomolecular perturbations have been traditionally performed using “classical” molecular or analytical methods, which evaluate one or few genes or their products at a time. Recent technical developments together with the increasing availability of high-throughput/content methodologies have enabled a more comprehensive overview of these molecular alterations and thus provide new approaches to the diagnosis and/or treatment of this group of disorders. Herein, we will review recent evidence pointing to the capacity of the so-called omics techniques in studying the nervous system infections with an emphasis on genomics, transcriptomics, and proteomics technologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
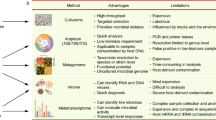
The increasing availability of the “Omics” technologies has ushered in a new era in the fields of microbiology and infectious diseases. High-throughput, high-content genomics, transcriptomics, proteomics, and metabolomics methods, combined with robust bioinformatics and computational tools have enabled researchers to obtain information about quantities and interactions of different biomolecules at system and whole organism levels [1, 2]. When applied to host–microbe interactions, these methods can provide unparalleled information about the complex nature of microbial infections’ pathophysiology [3, 4]. In the field of infectious diseases, neurological infections have been a particularly important area of research and clinical care, partly due to their high morbidity and mortality rates but also due to immense challenges inherent in establishing a diagnosis and subsequent management of the central nervous system (CNS) infections. These obstacles stem from the complexities in disease presentation, relative inaccessibility of tissues, and the limited CNS penetration of some anti-microbial/viral therapies.
In this review, we will focus on the applications of the omics methods in the diagnosis and pathogenesis of neurological infections. Given the interconnected nature of biomolecular networks, the information derived from different omics methods can be integrated to provide a complete view of the system (e.g., infectious agent and host responses) under investigation. Indeed, biomedical researchers use several omics platforms simultaneously to gain more detailed and accurate insights into the biological system(s) under investigation. Nonetheless, rather than discussing different types of omics information for individual neurological infections, in this review, we will examine the available information in separate genomics, transcriptomics, and proteomics sections. In each section, the findings of omics studies for major neuropathogenic microorganisms, including viruses, bacteria, and prions will be reviewed.
Genomics Studies and Neurological Infections
Unlike the traditional Sanger sequencing in which nucleic acid sequencing was limited to few DNA samples at a time, the so-called next generation sequencing (NGS) methods have the capability to sequence millions of sequences simultaneously and expediently [5]. The ability to sequence the entire nucleic acid content (DNA and RNA) of human tissues, which is a highly complex mixture of human nucleic acids as well as that of microbial flora and microbial pathogens, is transforming the way microbes and host–microbe interactions are detected and investigated [6]. Indeed, before the advent of high-throughput sequencing, detection of microbial pathogens was almost entirely based on traditional Koch’s postulates, which required isolation and culture of the microbes. Definition of natural microbial flora was similarly dependent on microbiological or low-throughput molecular methods. While the field is still in its initial stages, in this section, we will summarize some of the recent findings of “genomic” studies on nervous system-microbe interactions.
Genomic Studies on Viral Infections of the Nervous System
Viral infections of the nervous system have been a focus of medical research for generations both because of direct morbidity and mortality caused by neurovirulent viruses and due to potential association of viral infections with other nervous system disorders including neuroinflammatory disorders. NGS methods have been used both for detection of viral pathogens when classical virology or molecular methods have failed and for investigating the association of viruses with other neurological entities.
Identifying viral agents that might be responsible for encephalitis of unknown etiology represents a classical example of the application of NGS methods to neurological infections. There are a few reports highlighting the potential diagnostic value of NGS in this area. Using deep sequencing, Chan et al. successfully identified viral agents in some autopsy brain tissues from cases of encephalitis with unknown cause [7]. Using NGS analysis followed by confirmatory tests, they identified herpes virus-1 and measles virus in three encephalitis cases. In a similar study, using NGS analysis of a brain biopsy tissue, Naccache et al. were able to identify a neuroinvasive astrovirus as the cause of a fatal case of progressive encephalitis in an immunocompromised patient [8]. Likewise, through high-throughput sequencing of randomly amplified cerebrospinal fluid (CSF) nucleic acid sequences, Benjamin et al. were able to identify parvovirus 4 genome in CSF samples from pediatric cases of encephalitis with unknown etiology [9]. In addition to human samples, NGS methods have been used to survey for the presence of known viruses or evolution of new viruses in arthropod vectors as well as veterinary samples [10]. Even when the viral etiology is known through classical molecular techniques, NGS methods can be used to detect the viral strain, as well as transcriptomics changes, following infection with neurovirulent viruses. Massive parallel sequencing performed on brain tissues derived from a patient with rabies virus infection by our group showed numerous sequence tags corresponding to different regions of silver-haired bat-associated RV (SHBRV) strain 18 of rabies virus, a finding that was consistent with patient’s medical history [11].
With the emergence of retroviral infections of the nervous system in late twentieth century, there has been a resurgence of interest in neurovirology and in applying more sophisticated molecular methodologies in understandings them, considering the more subtle nature of retrovirus–host interactions [12]. HIV-1 and HTLV-1 are the two major retroviruses in humans, both of which infect the nervous system and cause neurological disorders. The majority of omics studies in the context of retroviral infections of the nervous system have focused on transcriptomic analyses (see below). Nonetheless, some research effort has been put into analyzing the impact of retroviral infection and associated immunosuppression on microbial agents in the brain. In a study by our group, we analyzed the impact of HIV infection on host brain microbiome using NGS methods [13•]. HIV infection of the brain is characterized by microglial and astrocyte activation and the induction of numerous proinflammatory cytokines and mediators. Nonetheless, sequence tags derived from the brains of HIV-infected individuals showed a preponderance of human endogenized retroviral RNA sequence elements belonging to the human endogenous retrovirus-K (HERV-K (II)) family compared to uninfected individuals that were chiefly located in neurons and subsequently shown to exert neuroprotective effects [14].
Aside from the identification of viral etiologies for encephalitis, researchers have harnessed the strength of NGS in pathogen detection to test the hypotheses regarding the association of viruses with other neurological disorders. One interesting example is the controversial association between viruses and neurological malignancies, including the aggressive brain tumor, glioblastoma [15, 16]. The presence of CMV genomic DNA and transcribed mRNAs has been reported in glioblastoma samples [15]. CMV antigens have also been detected in these samples by some laboratories but not others [15]. Having important therapeutic implications, unbiased next generation sequencing has been recently applied to glioblastoma samples to find a definitive answer. In one study, high coverage DNA sequences of 34 glioblastoma samples failed to detect CMV DNA in surgically resected tumors [16]. In another study on high-grade glioma samples, NGS detected viral sequences belonging to EBV and roseolovirus (HHV-6 and HHV-7) but not human CMV in the samples [17]. Another NGS study of glioblastoma samples reported an anti-viral-like type I interferon response in some samples, yet it failed to detect any viruses [18]. Neuroinflammatory diseases are another group of neurological disorders whose association with viruses has been speculated for a long time. Multiple sclerosis (MS) is an important neuroinflammatory disease, which is caused by autoimmune inflammation directed against myelin antigens. While molecular and immunological data have pointed to association with infectious agents, including members of the herpesvirus family and endogenous retroviruses, the issue remains highly controversial [19, 20]. Several groups have used NGS to detect viral genomes in multiple sclerosis tissues or CSF. In an interesting study, Kriesel et al. performed deep sequencing of RNA extracted from 50 frozen MS brain samples as well control tissues. After subtracting sequence reads related to human transcripts, they were able to detect an overrepresentation of sequence belonging to 12 different viral taxa in some of the MS samples [21]. These included sequences from GBV-C, a virus not previously associated with MS. Interestingly, sequence hit rate was not significantly different between MS and control cases for several viral taxa that were previously associated with MS, i.e., CMV, HHV-6, EBV, and measles virus [21, 22]. In another study, Schmitt et al. used high-throughput amplicon sequencing to perform a comprehensive analysis of the transcription of HERV-W loci in MS brains. The researchers were able to identify transcription from more than 100 HERV-W loci in the brains of MS patients [23]. Our group reported that there are multiple loci of transcription for the Syncytin-1 (envelope) gene encoded by HERV-W but 7q21.2 was the principal locus using a combination of conventional and NGS approaches [24]. In summary, NGS has provided new insights into identities and actions of ancient and current viruses in the nervous system.
Genomic Studies on Bacterial Infections of the Nervous System
NGS methods have been used to investigate multi-agent bacterial infections of the CNS. In an interesting work by Kommedal et al., the investigators used NGS methods to analyze bacterial species derived from brain abscesses in 52 surgical samples. Using an ion torrent NGS method, the researchers generated an average of 50,000 sequence reads of amplified 16 s bacterial RNA per sample [25]. This method was able to detect threefold more bacterial species compared with conventional culture methods, highlighting the capacity of high-throughput genomics in unraveling the complex nature of polymicrobial brain infections. In another study, unbiased NGS was used to detect a potential pathogen in a case of meningoencephalitis in which all pathogen-specific PCRs and infectious disease investigations on plasma, urine, CSF, as well as brain biopsy yielded negative results [26•]. NGS performed on the nucleic acid extracted from the CSF of the patient detected sequence reads corresponding to a leptospira species, a finding that was later confirmed by PCR. This led to a diagnosis of neuroleptospirosis, which was successfully treated [26•].
Similar to viral infections, some bacterial infections have also been reported to be associated with nervous system autoimmune and inflammatory disorders. One key example is the reported association between Campylobacter jejuni (C. jejuni) enteric infections with Guillain–Barré syndrome (GBS) [27, 28]. Considered to be a result of “molecular mimicry” associated with particular strains of the bacterium, comparative genomic analysis methods have been used to gain information about the strain of C. jejuni in GBS cases. In a study by Taboada et al., researchers used comparative genomic hybridization to identify isolates of C. jejuni, which show an association with GBS [29]. Altogether, these studies illustrate the ability of high-throughput sequencing to generate information about microbial etiology or associations with neurological disease, which is beyond the grasp of traditional molecular methodologies.
Transcriptomics Studies and Neurological Infections
Transcriptomic analyses are the most frequently performed type of omics research in neurological infections. As a general rule, host–microbe interactions lead to altered gene expression in host tissues, which could be detected by classical low-throughput gene expression analysis methods or high-throughput technologies including microarrays and RNA sequencing. While detection of altered gene expression might facilitate the diagnosis of neurological infections, transcriptomic methods have been chiefly used to gain insight into the molecular pathogenesis of neurological infections. Transcriptomic studies have been reported in numerous viral and bacterial infections of both central and peripheral nervous systems with viral infections of the CNS attracting more attention largely due to the emergence (or resurgence) of neurotropic viruses in the last few decades.
Transcriptomic Studies on Viral Infections of the Nervous System
HIV-associated neurological disorders are a group of nervous system diseases which occur as a consequence of HIV-1 infection of nervous system. HIV-associated neurocognitive disorder (HAND) is a neurocognitive syndrome, which results from direct infection of brain macrophages including microglia and trafficking macrophages by the virus, prompted by infiltration of infected monocytoid cells into the CNS [30]. Unlike many neurotropic viruses, HIV does not infect neurons and displays minimal productive infection of astrocytes. Nonetheless, synaptic injury and eventual neuronal apoptosis are key neuropathological features of HAND. While the HIV genome encodes proteins with direct neurotoxic properties, it is also widely accepted that transcriptional changes induced by HIV in monocytoid cells and astrocytes lead to widespread neuroinflammation with adverse effects on neuronal viability and survival [12]. Transcriptomic studies carried out by different groups have helped elucidate neuropathogenic mechanisms activated in HIV-infected brain tissue. One of the first studies was performed by Roberts et al., using the frontal lobe tissue of SIV-infected monkeys, a primate model for NeuroAIDS. They identified 98 genes including cell cycle and signaling and proteinase inhibitor genes, which showed upregulation in the brain following SIV infection [31]. Likewise, transcriptomic analysis of human brain tissue infected with HIV has shown dysregulation of numerous genes in HIV-infected brain, including increased levels of anti-viral and immune response-related gene and diminished levels of transcripts associated with synaptic transmission and neurogenesis [32]. Of note, brain transcript profiling of patients receiving anti-retroviral therapy demonstrated significant reduction in gene expression dysregulation in treated individuals [32]. Transcriptional profiling has also been performed on neural cells exposed to HIV or its proteins [33–37]. Interestingly, a meta-analysis comparing the results of transcript profiling in HAND, Alzheimer’s disease, and multiple sclerosis reported similarities in gene dysregulation between these three neurodegenerative disorders, which underlines the activation of similar neuropathogenic and/or neuroprotective mechanisms in CNS despite various etiologies [38].
West Nile virus (WNV) infection of the nervous system is another example of an emerging neurological infection [39]. A flavivirus, WNV infection of the nervous system can manifest as encephalitis, meningitis, or myelitis [40, 41]. Transcriptomic studies of WNV have been performed in WNV-infected neural cells or CNS tissues derived from WNV-infected animals. In a study by Koh et al., researchers analyzed the transcriptome of human glioblastoma cells exposed to the virus and found upregulation of different groups of transcripts including transcripts involved in interferon responses and apoptosis [42]. Interestingly, they also observed suppression of several mitochondrial genes involved in energy metabolism in infected cells [42]. Another study of WNV-infected mouse brains also found enhanced levels of interferon response genes and apoptosis in brain tissues infected with highly neuroinvasive WNV strains [43].
Transcript profiling techniques have been applied to detect gene expression changes in another flavivirus-mediated neurological infection, Japanese encephalitis virus (JEV). Microarray profiling of transcripts derived from JEV-infected neuroblastoma cells by Gupta et al. found induction of apoptosis and anti-viral response genes as well as chemokines [44]. Whole genome sequencing of JEV-infected mouse brains by the same group produced differential expression of pattern recognition receptors and components of inflammasome as well as chemotactic factors following virus infection [45]. Similar findings indicating the induction of pattern recognition receptors in mouse neurons and brains tissue following JEV infection has been reported by other groups [46]. In a remarkable study by Clarke et al., researchers compared transcriptomic changes caused by three neurotropic viruses, i.e., WNV, JEV, and reovirus in mouse brain [47]. Consistent with previous studies, genes related to interferon responses and neuroinflammation as well as cell death were among the altered genes. The two flaviviruses, WNV and JEV, but not the reovirus, induced the expression of tRNA synthetases while all three viruses suppressed genes associated with glutamate signaling [47].
Host and Pathogen Transcriptomic Studies on Bacterial Infections of the Nervous System
Transcriptomic analysis of bacterial infections of the nervous system can be categorized into two groups; one group focuses on brain’s transcriptomic changes following exposure to the bacteria while the other group concentrates on the bacterial transcriptional response during infection. As is the case for viral infections, host transcriptomic analyses aim to unravel both the neuroprotective or neuropathogenic molecular events at the RNA level. Using an ex vivo model of Lyme disease, Ramesh et al. applied DNA microarrays to assess the transcriptional response of primate brain cortical tissue following exposure to spirochetes. Altered expression of transcripts regulating inflammation, oligodendrocyte, and neuronal apoptosis was detected in the cortical tissues [48]. In a related study, researchers used complementary DNA (cDNA) microarrays to evaluate transcriptional changes in microglia and astrocytes following exposure to Borrelia burgdorferi in the presence of tetracycline antibiotics in culture [49]. Microarrays have also been used to assess the pathophysiology of bacterial meningitis at the transcriptomic level. Of interest, the response of human brain microvascular endothelial cells (hBMECs) to bacteria has been investigated by different groups. Banerjee et al. used microarrays to assess hBMECs response to pneumococci [50], and Wang et al. studied the response to Listeria monocytogenes [51].
In addition to host responses, transcriptomic studies focusing on the bacterial transcripts have provided information of applied importance. In a seminal study by Mahdi et al., researchers tried to identify pneumococcal virulence genes preferentially expressed during meningitis. Using a mouse model of pneumococcal meningitis, differential expression of 34 bacterial genes was identified. One differentially expressed gene, α-glycerophosphate oxidase (GlpO), showed cytotoxicity for microvascular cells and mutant bacteria lacking the gene failed to adhere to brain microvascular endothelia in vitro or cause meningitis in vivo. Immunization of mice with GlpO protected the animals against invasive infection [52•]. Such studies show the power of transcriptomic analyses in identifying candidates for vaccine development. In a similar study, transcriptional changes in Neisseria meningitides have been evaluated following the interaction of bacterium with epithelial cells and endothelial cells, representing two key stages of meningococcal infection [53]. Likewise, expression profiling of Mycobacterium tuberculosis genes has demonstrated transcriptomic changes within the mycobacterium during invasion of brain microvascular endothelial cells [54].
Transcriptomic Studies on Prion Infections
Prion infections of the CNS represent unique neurological infections because the infectious agent lacks nucleic acids but it alters host-encoded protein (PrP) conformation resulting in a pathogenic infectious agent. While prion agents cannot be subjected to NGS studies for the detection of pathogen because the encoding nucleic acids are host-derived, numerous host transcriptomic studies have been performed to investigate gene expression alterations following prion infections (reviewed in [55]). Similar to viral infections of the nervous system, transcriptional profiling has been employed chiefly to gain insights into the pathogenic mechanisms that lead to neurodegeneration during prion infection. In an interesting study, Crespo et al. identified a group of genes related to immune responses, which affect genes involved in prion replication and also neural cell death [56]. Bach et al. performed a transcriptional profiling on prion-infected neuronal cells and identified the induction of cholesterol synthesis-associated transcripts following infection [57]. Transcriptional profiling of laser capture microdissected CA1 neurons has been used successfully to determine gene expression dysregulation at the preclinical stages of disease, when there is a potential for interrupting the neurodegenerative process [57]. Moreover, to detect genes that are associated with susceptibility to prion infection, Marbiah et al. analyzed the transcriptome of prion-resistant mutants of highly susceptible neuroblastoma cells, which led them to the detection of several genes with a role in extracellular matrix remodeling as potential determinants of cell susceptibility/resistance to prion infections [58].
Proteomic Studies and Neurological Infections
While transcriptional studies have shed light on altered gene expression following infection at the RNA level, proteomic analyses of infected neural tissues and cells can provide further information, not accessible at the RNA level, about the pathogenesis of neurological infections. Conventional proteomic studies which use techniques including 2-D gel electrophoresis followed by mass spectrometric analyses (2DG/MS) have been used to identify differentially expressed proteins in a variety of nervous system infections. Moreover, more advanced quantitative technologies have also made it feasible to look at protein quantities during infections. Indeed, this latter approach may represent a harbinger for new perspectives on the pathophysiology of neurological disorders including infectious diseases.
Proteomics Studies on Viral Infections of the Nervous System
Similar to transcriptomic studies, a substantial part of proteomic studies on neurovirulent viruses has focused on HIV-induced neurological disorders, particularly HAND. In an effort to identify potential CSF biomarkers for HAND, Rozek et al. performed a proteomic study using cerebrospinal fluid (CSF) and were able to detect altered levels of several proteins including vitamin D-binding protein, complement C3, and cystatin C in CSF from patients with HAND [59]. Another CSF proteomic study revealed lower levels of antioxidant enzymes in CSF samples from cases with HAND [60]. Proteomic analyses of CSF from SIV-infected monkeys displayed higher levels of serine proteases including complement C3 and plasminogen together with alpha1-antitrypsin in the CSF following SIV infection [61]. Indeed, alteration in the CNS’ protease–antiprotease network and the resulting variations in protein degradation have been implicated in HIV neuropathogenesis. Of interest, it has been shown previously that induction of a matrix metalloproteinase in HIV-infected macrophages leads to generation of chemokine cleavage products with significant neurotoxic properties [62].
In addition to CSF studies, several groups have undertaken proteome-level analyses on HIV-infected postmortem brain tissues or neural cells exposed to the virus or its proteins. Gelman et al. performed a focused proteomic study on the synaptosomes isolated from frontal cortex of HIV-encephalitis cases. Results showed lower levels of synapsin1 and stathmin proteins and enhanced levels of 14-3-3zeta and 14-4-4epsilon proteins in HIV-infected brains [63]. 2DG/MS analysis of HIV Tat-expressing astrocytes showed altered levels of numerous proteins including cytoskeletal and signaling molecules in Tat-expressing cells [64]. HIV-infected macrophages have been demonstrated to affect brain microvascular endothelial cells’ proteome, a phenomenon which might be related to perturbed BBB permeability during HAND [65]. HTLV-1 is another retrovirus which is capable of inducing neurological disorders, i.e., HTLV-1 associated myelopathy (HAM), also termed tropical spastic paraparesis (TSP). Proteomic analysis of CSF and plasma samples derived from HAM/TSP cases has identified several proteins, which could serve as potential biomarkers for disease [66].
Other neurotropic viruses including rabies have also been subject of proteomic studies. 2DG/MS proteomic analysis on postmortem human brain tissues infected with rabies virus showed altered levels of proteins related to cytoskeleton, metabolism, proteasome, and immune regulation [67]. Altered expression of cytoskeletal proteins have also been reported in 2DG/MS studies in neuronal cell lines infected with different strains of rabies virus [68]. Using iTRAQ quantitative proteomic method, Venugopal et al. detected overexpression of functionally diverse proteins including karyopherin alpha 4 (KPNA4), calcium calmodulin-dependent kinase 2 alpha (CAMK2A), and glutamate ammonia ligase (GLUL) in postmortem rabies brains [69]. Likewise, protein profiling of mouse brains infected with rabies has shown differential expression of proteins involved in ion homeostasis and synaptic function [70].
Proteomics Studies on Bacterial Infections of the Nervous System
Proteomic studies on bacterial infections of the nervous system have targeted different aspects of neuro-bacterial infections, including identification of vaccine targets, discovering bacterial virulence factors, and neuropathogenic mechanisms as well as rapid pathogen detection.
Detecting immunodominant proteins belonging to a pathogen is an active area of work in infectious disease proteomics, an area of research which is labeled “immunoproteomics” [71, 72]. Among bacteria which can cause neurological infections, N. meningitides has been subject of several immunoproteomic studies. A chief aim of these studies has been to detect parts of the meningococcal proteome that can induce a protective immune response against the pathogen. In a study by Tsolakos et al., researchers used proteomic analysis of N. meningitides type b (NMB) surface antigens to detect proteins which could be recognized by antibodies in the sera of MNB-immunized animals [73•]. Using this method, they were able to identify several new immunogenic proteins which are expressed at the surface of the bacterium [73•]. In another study, Williams et al. used an immunoproteomic approach to identify Neisseria antigens which could evoke cross-reactive antibody responses against different Neisseria strains and hence could serve as potential candidates for new meningococcal vaccines [74]. Similarly, Mendum et al. tried to identify Neisseria antigens which lead to cross-protective humoral immune responses in patients recovering from meningococcal infections [75].
Proteomics methods are making unprecedented contributions to the study of bacterial virulence factors [76, 77]. Application of quantitative proteomic methods to microbial proteins allows for organism-level determination of molecules which are expressed in virulent but not nonvirulent strains of a bacterium. This has provided microbiologists with a powerful tool for virulence factor identification. There are multiple reports of using proteomic techniques to detect virulence factors in major bacterial pathogens including Staphylococcus aureus, M. tuberculosis, and Yersinia pestis. In the context of neurological infections, proteomic-based investigations of virulence factors have been performed for N. meningitides. Gault et al. used proteomic methods to examine posttranslational modifications in Neisseria virulence factor protein, pilin [78]. Ferrari et al. have used proteomics methods to study outer membrane vesicles (OMV), bacterial-derived elements which are necessary for bacterial survival and pathogenesis [79]. In addition to examining cytoplasmic or cell surface proteins, virulence factor discovery has been accomplished through the so-called secretome analysis. This type of research applies proteomic methods to evaluate the complex set of molecules which are secreted by bacteria in culture or in infected tissues. The secreted bacterial proteins could act as virulence factors either by acting as a toxin or toxin transporters or by inhibiting immune responses [77]. Of interest, by comparing the secretome of virulent and nonvirulent strains of Cryptococcus, Campbell et al. identified proteins, which could enhance the neuroinvasiveness of a strain of these fungi [80]. Comparative secretome analysis has also been performed on secreted proteins from pathogenic N. meningitides [81].
Besides making contributions to vaccine design and virulence factor discovery, proteomic methods are revolutionizing the practice of clinical microbiology, where pathogen identification is a key objective [82]. In particular, matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI)-TOF has proven to be a fast and reliable method for identification of bacteria in clinical samples [83]. That said, examples of proteomic-based pathogen identification in neurological infections are just emerging. In a report by Segawa et al., researchers used MALDI-TOF analysis of the CSF from a case of bacterial meningitis to detect Klebsiella pneumonia as a causative agent [84]. In another study, MALDI-TOF analysis of spinal fluid was successfully used to accurately detect the pathogen in a case of pneumococcal meningitis [85]. In addition to mass spectrometric analyses, protein microarrays generated by recombinant bacterial proteins have also been used to screen the sera of patients with meningococcal meningitis to identify potential diagnostic markers for disease [86]. Moreover, 2-DG/MS analysis of CSF as well as quantitative proteomics on brain tissue was employed for biomarker discovery in cases of tuberculous meningitis [87, 88].
Proteomic Studies on Prion Infections
Prion infections of nervous system have been subject of intense investigation by proteomic methods. Proteomic analyses have been used to address varieties of questions about prion disease, including structural analysis of prion proteins, biomarker discovery, detection of interacting molecules, and proteome dysregulation following infection [89].
In a remarkable study on human brain tissues, protein profiling of cortical and cerebellar tissues of Creutzfeldt–Jakob disease (CJD) and FFI cases by Shi et al. revealed both induction and suppression of numerous proteins in the affected brains. Pathways related to Parkinson’s disease, Alzheimer’s disease, oxidative phosphorylation, lysosome, protein export, and cytochrome P450 were among the affected pathways [90•]. Proteomic analysis of prion-infected mouse brain by Moore et al. showed dysregulation of proteins related to neuroinflammatory response and complement activation as well as cell death and metabolism [91]. In addition to in vivo analyses, in vitro systems in which neuronal cell lines are exposed to infectious prion particles have provided further information in proteomic studies. Of note, modified expression of cellular chaperone proteins including chaperones Grp78/BiP, protein disulfide-isomerase A6, Grp75, and Hsp60 has been reported in neuroblastoma cells exposed to prions, a finding which is consistent with induction of cellular protein misfolding by infectious prions [92]. In another line of studies, high-throughput protein interaction experiments have been performed to detect prion protein interacting proteins (PrPIPs) in cells. Using protein microarrays, Satoh et al. were able to identify 47 novel PrPIPs which were mainly involved in nucleic acid recognition [93]. Using 2-DE followed by mass spectrometry, Storm et al. also detected proteins involved in nucleic acid metabolism to interact with Prp (C) [94].
Several research groups have tried to use proteomic methods to discover biomarkers for prion disease. Creutzfeldt–Jakob disease (CJD) has been of particular interest in this area, considering that a definite diagnosis can only be made by postmortem analysis of the brain [95]. More than 15 years ago, 2D gel analysis of CSF from CJD patients led to the detection of 14-3-3 proteins as a disease biomarker [96]. Further 2D gel studies of CSF from CJD patients followed by mass spectrometric identification of proteins have later added other potential biomarkers to the list, including neuron-specific enolase, and lactate dehydrogenase in the sporadic CJD cases [97]. More recently, surface-enhanced laser desorption/ionization (SELDI)-TOF analysis of CSF from CJD and control cases has identified increased levels of ubiquitin and cystatin C in the CSF of these patients [98, 99]. Other potential biomarkers including fatty acid binding protein, FABP, have also been proposed by proteomic studies [100].
Conclusions and Future Perspectives
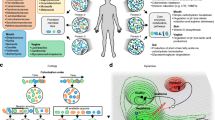
The advent of omics technologies has prompted the elucidation of the remarkable complexity of microbial agents and associated host responses in the CNS. Indeed, it is generally assumed that the nervous system is a sterile system/organ, devoid of any microbial flora unless a pathogenic agent entered and infected the CNS. This assumption has been challenged by NGS studies on brain-derived nucleic acids. Over the past three decades, multiple studies have shown that bacterial and viral proteins and nucleic acid sequences can be detected in human brain tissues in the absence of overt pathogenic CNS infections [101–104]. In a study by our group, massive parallel sequencing without prior amplification was performed on the cDNA derived from surgical brain resections and autopsied brain samples, which showed the presence of sequence tags belonging to different bacterial and viral species [13•]. Among bacteria, alpha-proteobacteria showed the highest frequency of sequence tags in both surgical resections and autopsy samples, a finding that was confirmed by amplifying the 16 s rRNA sequences from the samples [13•]. Similar findings in terms of microbial diversity were observed in the brains of SIV-infected macaques. These studies underscore the microbial diversity in the brain but also provide insight into the limitations of conventional technologies used for diagnosing and treating CNS infections.
There are other surfacing technologies that will contribute to the understanding of CNS infections including assays of microRNAs and metabolic products (“metabolomics”). Ongoing efforts to apply high-throughput analyses to microRNA expression in brain and plasma represent exciting opportunities to gain insights in disease pathogenesis and perhaps establish new biomarkers for disease [105–108]. Similarly, metabolomics approaches using nuclear magnetic resonance and mass spectrometry [11, 109] have been applied successfully to studying neurological infections and herald a new epoch in diagnosing and understanding these devastating diseases.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Aderem A. Systems biology: its practice and challenges. Cell. 2005;121(4):511–3.
Liu ET. Systems biology, integrative biology, predictive biology. Cell. 2005;121(4):505–6.
Thiele I, Heinken A, Fleming RM. A systems biology approach to studying the role of microbes in human health. Curr Opin Biotechnol. 2013;24(1):4–12.
van Baarlen P, Kleerebezem M, Wells JM. Omics approaches to study host-microbiota interactions. Curr Opin Microbiol. 2013;16(3):270–7.
Metzker ML. Sequencing technologies—the next generation. Nat Rev Genet. 2010;11(1):31–46.
Vayssier-Taussat M et al. Shifting the paradigm from pathogens to pathobiome: new concepts in the light of meta-omics. Front Cell Infect Microbiol. 2014;4:29.
Chan BK et al. Deep sequencing to identify the causes of viral encephalitis. PLoS One. 2014;9(4), e93993.
Naccache SN et al. Diagnosis of neuroinvasive astrovirus infection in an immunocompromised adult with encephalitis by unbiased next-generation sequencing. Clin Infect Dis. 2015;60(6):919–23.
Benjamin LA et al. Human parvovirus 4 as potential cause of encephalitis in children. India Emerg Infect Dis. 2011;17(8):1484–7.
Coffey LL et al. Enhanced arbovirus surveillance with deep sequencing: identification of novel rhabdoviruses and bunyaviruses in Australian mosquitoes. Virology. 2014;448:146–58.
Reinke SN et al. Metagenomic and metabolomic characterization of rabies encephalitis: new insights into the treatment of an ancient disease. J Infect Dis. 2013;207(9):1451–6.
Power C. Retroviral diseases of the nervous system: pathogenic host response or viral gene-mediated neurovirulence? Trends Neurosci. 2001;24(3):162–9.
Branton WG et al. Brain microbial populations in HIV/AIDS: alpha-proteobacteria predominate independent of host immune status. PLoS One. 2013;8(1):e54673. This study provided evidence about the impact of HIV infection of the brain on brain’s microbial diversity as well as the composition of microbes in uninfected human brain.
Bhat RK et al. Human endogenous retrovirus-K (II) envelope induction protects neurons during HIV/AIDS. PLoS One. 2014;9(7), e97984.
Lawler, S.E., Cytomegalovirus and glioblastoma; controversies and opportunities. J Neurooncol, 2015.
Tang KW, Hellstrand K, Larsson E. Absence of cytomegalovirus in high-coverage DNA sequencing of human glioblastoma multiforme. Int J Cancer. 2015;136(4):977–81.
Cimino PJ et al. Detection of viral pathogens in high grade gliomas from unmapped next-generation sequencing data. Exp Mol Pathol. 2014;96(3):310–5.
Cosset E et al. Comprehensive metagenomic analysis of glioblastoma reveals absence of known virus despite antiviral-like type I interferon gene response. Int J Cancer. 2014;135(6):1381–9.
Gilden DH. Infectious causes of multiple sclerosis. Lancet Neurol. 2005;4(3):195–202.
Scarisbrick IA, Rodriguez M. Hit-hit and hit-run: viruses in the playing field of multiple sclerosis. Curr Neurol Neurosci Rep. 2003;3(3):265–71.
Kriesel JD et al. Deep sequencing for the detection of virus-like sequences in the brains of patients with multiple sclerosis: detection of GBV-C in human brain. PLoS One. 2012;7(3), e31886.
Virtanen JO, Jacobson S. Viruses and multiple sclerosis. CNS Neurol Disord Drug Targets. 2012;11(5):528–44.
Schmitt K et al. Comprehensive analysis of human endogenous retrovirus group HERV-W locus transcription in multiple sclerosis brain lesions by high-throughput amplicon sequencing. J Virol. 2013;87(24):13837–52.
Bhat RK et al. Age- and disease-dependent HERV-W envelope allelic variation in brain: association with neuroimmune gene expression. PLoS One. 2011;6(4), e19176.
Kommedal O et al. Massive parallel sequencing provides new perspectives on bacterial brain abscesses. J Clin Microbiol. 2014;52(6):1990–7.
Wilson MR et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370(25):2408–17. This study represents a good example of the clinical applications of NGS in identifying the pathogens underlying neurological infections, when other diagnostic methods do not lead to a conclusive diagnosis.
Yuki N. Molecular mimicry between gangliosides and lipopolysaccharides of campylobacter jejuni isolated from patients with Guillain-Barre syndrome and Miller Fisher syndrome. J Infect Dis. 1997;176 Suppl 2:S150–3.
Tsang RS. The relationship of campylobacter jejuni infection and the development of Guillain-Barre syndrome. Curr Opin Infect Dis. 2002;15(3):221–8.
Taboada EN et al. Comparative genomic analysis of campylobacter jejuni associated with Guillain-Barre and Miller Fisher syndromes: neuropathogenic and enteritis-associated isolates can share high levels of genomic similarity. BMC Genomics. 2007;8:359.
Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5(1):69–81.
Roberts ES et al. Induction of pathogenic sets of genes in macrophages and neurons in NeuroAIDS. Am J Pathol. 2003;162(6):2041–57.
Borjabad A et al. Significant effects of antiretroviral therapy on global gene expression in brain tissues of patients with HIV-1-associated neurocognitive disorders. PLoS Pathog. 2011;7(9), e1002213.
Albright AV, Gonzalez-Scarano F. Microarray analysis of activated mixed glial (microglia) and monocyte-derived macrophage gene expression. J Neuroimmunol. 2004;157(1–2):27–38.
Boven LA et al. Brain-derived human immunodeficiency virus-1 Tat exerts differential effects on LTR transactivation and neuroimmune activation. J Neurovirol. 2007;13(2):173–84.
Galey D et al. Differential transcriptional regulation by human immunodeficiency virus type 1 and gp120 in human astrocytes. J Neurovirol. 2003;9(3):358–71.
Kim SY et al. Microarray analysis of changes in cellular gene expression induced by productive infection of primary human astrocytes: implications for HAD. J Neuroimmunol. 2004;157(1–2):17–26.
Wang Z et al. Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: potential role in neuropathogenesis. J Neurovirol. 2004;10 Suppl 1:25–32.
Borjabad A, Volsky DJ. Common transcriptional signatures in brain tissue from patients with HIV-associated neurocognitive disorders, Alzheimer’s disease, and multiple sclerosis. J Neuroimmune Pharmacol. 2012;7(4):914–26.
Petersen LR, Roehrig JT. West Nile virus: a reemerging global pathogen. Emerg Infect Dis. 2001;7(4):611–4.
Jeha LE et al. West Nile virus infection: a new acute paralytic illness. Neurology. 2003;61(1):55–9.
Sejvar JJ. Clinical manifestations and outcomes of West Nile virus infection. Viruses. 2014;6(2):606–23.
Koh WL, Ng ML. Molecular mechanisms of West Nile virus pathogenesis in brain cell. Emerg Infect Dis. 2005;11(4):629–32.
Venter M et al. Gene expression in mice infected with West Nile virus strains of different neurovirulence. Virology. 2005;342(1):119–40.
Gupta N et al. Chemokine profiling of Japanese encephalitis virus-infected mouse neuroblastoma cells by microarray and real-time RT-PCR: implication in neuropathogenesis. Virus Res. 2010;147(1):107–12.
Gupta N, Rao PV. Transcriptomic profile of host response in Japanese encephalitis virus infection. Virol J. 2011;8:92.
Fadnis PR et al. Innate immune mechanisms in Japanese encephalitis virus infection: effect on transcription of pattern recognition receptors in mouse neuronal cells and brain tissue. Viral Immunol. 2013;26(6):366–77.
Clarke P et al. Virus-induced transcriptional changes in the brain include the differential expression of genes associated with interferon, apoptosis, interleukin 17 receptor A, and glutamate signaling as well as flavivirus-specific upregulation of tRNA synthetases. MBio. 2014;5(2):e00902–14.
Ramesh G et al. Interaction of the Lyme disease spirochete Borrelia burgdorferi with brain parenchyma elicits inflammatory mediators from glial cells as well as glial and neuronal apoptosis. Am J Pathol. 2008;173(5):1415–27.
Bernardino AL, Kaushal D, Philipp MT. The antibiotics doxycycline and minocycline inhibit the inflammatory responses to the Lyme disease spirochete Borrelia burgdorferi. J Infect Dis. 2009;199(9):1379–88.
Banerjee A et al. Activation of brain endothelium by pneumococcal neuraminidase NanA promotes bacterial internalization. Cell Microbiol. 2010;12(11):1576–88.
Wang C et al. Early gene response of human brain microvascular endothelial cells to Listeria monocytogenes infection. Can J Microbiol. 2011;57(5):441–6.
Mahdi LK et al. Identification of a novel pneumococcal vaccine antigen preferentially expressed during meningitis in mice. J Clin Invest. 2012;122(6):2208–20. This study is an excellent example of the power of “Omics” analyses in identifying bacterial neurovirulence factors and vaccine candidates.
Dietrich G et al. Transcriptome analysis of Neisseria meningitidis during infection. J Bacteriol. 2003;185(1):155–64.
Jain SK et al. Mycobacterium tuberculosis invasion and traversal across an in vitro human blood–brain barrier as a pathogenic mechanism for central nervous system tuberculosis. J Infect Dis. 2006;193(9):1287–95.
Basu U, Guan LL, Moore SS. Functional genomics approach for identification of molecular processes underlying neurodegenerative disorders in prion diseases. Curr Genomics. 2012;13(5):369–78.
Crespo I et al. Gene regulatory network analysis supports inflammation as a key neurodegeneration process in prion disease. BMC Syst Biol. 2012;6:132.
Bach C et al. Prion-induced activation of cholesterogenic gene expression by Srebp2 in neuronal cells. J Biol Chem. 2009;284(45):31260–9.
Marbiah MM et al. Identification of a gene regulatory network associated with prion replication. Embo J. 2014;33(14):1527–47.
Rozek W et al. Cerebrospinal fluid proteomic profiling of HIV-1-infected patients with cognitive impairment. J Proteome Res. 2007;6(11):4189–99.
Velazquez I et al. Antioxidant enzyme dysfunction in monocytes and CSF of Hispanic women with HIV-associated cognitive impairment. J Neuroimmunol. 2009;206(1–2):106–11.
Pendyala G et al. Cerebrospinal fluid proteomics reveals potential pathogenic changes in the brains of SIV-infected monkeys. J Proteome Res. 2009;8(5):2253–60.
Zhang K et al. HIV-induced metalloproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration. Nat Neurosci. 2003;6(10):1064–71.
Gelman BB, Nguyen TP. Synaptic proteins linked to HIV-1 infection and immunoproteasome induction: proteomic analysis of human synaptosomes. J Neuroimmune Pharmacol. 2010;5(1):92–102.
Pocernich CB et al. Proteomics analysis of human astrocytes expressing the HIV protein Tat. Brain Res Mol Brain Res. 2005;133(2):307–16.
Ricardo-Dukelow M et al. HIV-1 infected monocyte-derived macrophages affect the human brain microvascular endothelial cell proteome: new insights into blood–brain barrier dysfunction for HIV-1-associated dementia. J Neuroimmunol. 2007;185(1–2):37–46.
Ishihara M et al. A plasma diagnostic model of human T-cell leukemia virus-1 associated myelopathy. Ann Clin Transl Neurol. 2015;2(3):231–40.
Farahtaj F et al. Proteomics analysis of human brain tissue infected by street rabies virus. Mol Biol Rep. 2013;40(11):6443–50.
Zandi F et al. Expression changes of cytoskeletal associated proteins in proteomic profiling of neuroblastoma cells infected with different strains of rabies virus. J Med Virol. 2013;85(2):336–47.
Venugopal AK et al. Quantitative proteomics for identifying biomarkers for rabies. Clin Proteomics. 2013;10(1):3.
Dhingra V et al. Proteomic profiling reveals that rabies virus infection results in differential expression of host proteins involved in ion homeostasis and synaptic physiology in the central nervous system. J Neurovirol. 2007;13(2):107–17.
Fulton KM, Twine SM. Immunoproteomics: current technology and applications. Methods Mol Biol. 2013;1061:21–57.
Purcell AW, Gorman JJ. Immunoproteomics: mass spectrometry-based methods to study the targets of the immune response. Mol Cell Proteomics. 2004;3(3):193–208.
Tsolakos N et al. Identification of vaccine antigens using integrated proteomic analyses of surface immunogens from serogroup B Neisseria meningitidis. J Proteomics. 2014;101:63–76. This research demonstrates the success of “immuno-proteomics” approach in identifying the potential vaccine candidates for a neurovirulent bacterium.
Williams JN et al. Immuno-proteomic analysis of human immune responses to experimental Neisseria meningitidis outer membrane vesicle vaccines identifies potential cross-reactive antigens. Vaccine. 2014;32(11):1280–6.
Mendum TA et al. Towards the immunoproteome of Neisseria meningitidis. PLoS One. 2009;4(6), e5940.
Chao TC, Hansmeier N. The current state of microbial proteomics: where we are and where we want to go. Proteomics. 2012;12(4–5):638–50.
Wu HJ, Wang AH, Jennings MP. Discovery of virulence factors of pathogenic bacteria. Curr Opin Chem Biol. 2008;12(1):93–101.
Gault J et al. Complete posttranslational modification mapping of pathogenic Neisseria meningitidis pilins requires top-down mass spectrometry. Proteomics. 2014;14(10):1141–51.
Ferrari G et al. Outer membrane vesicles from group B Neisseria meningitidis delta gna33 mutant: proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics. 2006;6(6):1856–66.
Campbell, L.T., et al., Cryptococcus strains with different pathogenic potential have diverse protein secretomes. Eukaryot Cell, 2015.
van Ulsen P, Tommassen J. Protein secretion and secreted proteins in pathogenic Neisseriaceae. FEMS Microbiol Rev. 2006;30(2):292–319.
Clark AE et al. Matrix-assisted laser desorption ionization-time of flight mass spectrometry: a fundamental shift in the routine practice of clinical microbiology. Clin Microbiol Rev. 2013;26(3):547–603.
Nomura F. Proteome-based bacterial identification using matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS): a revolutionary shift in clinical diagnostic microbiology. Biochim Biophys Acta. 2015;1854(6):528–37.
Segawa S et al. Direct application of MALDI-TOF mass spectrometry to cerebrospinal fluid for rapid pathogen identification in a patient with bacterial meningitis. Clin Chim Acta. 2014;435:59–61.
Nyvang Hartmeyer G et al. Mass spectrometry: pneumococcal meningitis verified and Brucella species identified in less than half an hour. Scand J Infect Dis. 2010;42(9):716–8.
Steller S et al. Bacterial protein microarrays for identification of new potential diagnostic markers for Neisseria meningitidis infections. Proteomics. 2005;5(8):2048–55.
Kataria J et al. Two dimensional difference gel electrophoresis analysis of cerebrospinal fluid in tuberculous meningitis patients. J Proteomics. 2011;74(10):2194–203.
Kumar GS et al. Quantitative proteomics for identifying biomarkers for tuberculous meningitis. Clin Proteomics. 2012;9(1):12.
Moore RA, Faris R, Priola SA. Proteomics applications in prion biology and structure. Expert Rev Proteomics. 2015;12(2):171–84.
Shi Q et al. Proteomics analyses for the global proteins in the brain tissues of different human prion diseases. Mol Cell Proteomics. 2015;14(4):854–69. A comprehensive quantitative proteomic analysis of the cortex and cerebellum of prion-infected human brain.
Moore RA et al. Proteomics analysis of amyloid and nonamyloid prion disease phenotypes reveals both common and divergent mechanisms of neuropathogenesis. J Proteome Res. 2014;13(11):4620–34.
Provansal M et al. Proteomic consequences of expression and pathological conversion of the prion protein in inducible neuroblastoma N2a cells. Prion. 2010;4(4):292–301.
Satoh J et al. Protein microarray analysis identifies human cellular prion protein interactors. Neuropathol Appl Neurobiol. 2009;35(1):16–35.
Strom A et al. Identification of prion protein binding proteins by combined use of far-Western immunoblotting, two dimensional gel electrophoresis and mass spectrometry. Proteomics. 2006;6(1):26–34.
Qualtieri A et al. Proteomic profiling of cerebrospinal fluid in Creutzfeldt-Jakob disease. Expert Rev Proteomics. 2010;7(6):907–17.
Wiltfang J et al. Isoform pattern of 14-3-3 proteins in the cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. J Neurochem. 1999;73(6):2485–90.
Brechlin P et al. Cerebrospinal fluid-optimized two-dimensional difference gel electrophoresis (2-D DIGE) facilitates the differential diagnosis of Creutzfeldt-Jakob disease. Proteomics. 2008;8(20):4357–66.
Sanchez JC et al. Cystatin C as a potential cerebrospinal fluid marker for the diagnosis of Creutzfeldt-Jakob disease. Proteomics. 2004;4(8):2229–33.
Steinacker P et al. Ubiquitin as potential cerebrospinal fluid marker of Creutzfeldt-Jakob disease. Proteomics. 2010;10(1):81–9.
Steinacker P et al. Heart fatty acid binding protein as a potential diagnostic marker for neurodegenerative diseases. Neurosci Lett. 2004;370(1):36–9.
Schrijver IA et al. Bacterial peptidoglycan and immune reactivity in the central nervous system in multiple sclerosis. Brain. 2001;124(Pt 8):1544–54.
Sanders VJ et al. Detection of herpesviridae in postmortem multiple sclerosis brain tissue and controls by polymerase chain reaction. J Neurovirol. 1996;2(4):249–58.
Sanders VJ et al. Herpes simplex virus in postmortem multiple sclerosis brain tissue. Arch Neurol. 1996;53(2):125–33.
Brahic M et al. Detection of picornavirus sequences in nervous tissue of amyotrophic lateral sclerosis and control patients. Ann Neurol. 1985;18(3):337–43.
Gupta P et al. Genome-wide mRNA and miRNA analysis of peripheral blood mononuclear cells (PBMC) reveals different miRNAs regulating HIV/HCV co-infection. Virology. 2014;450–451:336–49.
Noorbakhsh F et al. MicroRNA profiling reveals new aspects of HIV neurodegeneration: caspase-6 regulates astrocyte survival. Faseb J. 2010;24(6):1799–812.
Witwer KW et al. A plasma microRNA signature of acute lentiviral infection: biomarkers of central nervous system disease. AIDS. 2011;25(17):2057–67.
Zhou L et al. A parallel genome-wide mRNA and microRNA profiling of the frontal cortex of HIV patients with and without HIV-associated dementia shows the role of axon guidance and downstream pathways in HIV-mediated neurodegeneration. BMC Genomics. 2012;13:677.
Cassol E et al. Cerebrospinal fluid metabolomics reveals altered waste clearance and accelerated aging in HIV patients with neurocognitive impairment. AIDS. 2014;28(11):1579–91.
Compliance with Ethics Guidelines
Conflict of Interest
Farshid Noorbakhsh, Atefeh Aminian, and Christopher Power declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Infection
Rights and permissions
About this article
Cite this article
Noorbakhsh, F., Aminian, A. & Power, C. Application of “Omics” Technologies for Diagnosis and Pathogenesis of Neurological Infections. Curr Neurol Neurosci Rep 15, 58 (2015). https://doi.org/10.1007/s11910-015-0580-y
Published:
DOI: https://doi.org/10.1007/s11910-015-0580-y