Abstract
Purpose of Review
Perinatal HIV-1 infection is associated with an increased risk for neurologic impairments. With limited access to clinical specimens, animal models could advance our understanding of pediatric central nervous system (CNS) disease and viral persistence. Here, we summarize current findings on HIV-1 CNS infection from nonhuman primate (NHP) models and discuss their implications for improving pediatric clinical outcomes.
Recent Findings
SIV/SHIV can be found in the CNS of infant macaques within 48 h of challenge. Recent studies show an impermeable BBB during SIV infection, suggesting neuroinvasion in post-partum infection is likely not wholly attributed to barrier dysfunction. Histopathological findings reveal dramatic reductions in hippocampal neuronal populations and myelination in infected infant macaques, providing a link for cognitive impairments seen in pediatric cases. Evidence from humans and NHPs support the CNS as a functional latent reservoir, harbored in myeloid cells that may require unique eradication strategies.
Summary
Studies in NHP models are uncovering early events, causes, and therapeutic targets of CNS disease as well as highlighting the importance of age-specific studies that capture the distinct features of pediatric HIV-1 infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
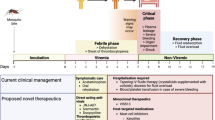
Globally, 1.7 million children are currently living with HIV-1 infection, with 160,000 new infections occurring annually [1]. The majority of new pediatric infections occur through mother-to-child transmission (MTCT) in utero, intrapartum, or post-partum through breast milk. While the mechanisms are not completely defined, HIV-1-infected infants experience a rapid progression of disease as compared to infected adults, with over 50% of HIV-1-infected children dying before the age of two in the absence of antiretroviral therapy (ART) [2]. ART has greatly reduced HIV-1-related morbidity and mortality but, alone, cannot purge the viral reservoir that is seeded early in infection. In the majority of HIV-1-infected individuals, interruption of ART leads to viral rebound, making daily adherence to medication a lifelong requirement to control virus replication. A handful of pediatric cases of prolonged ART-free remission have been reported, reflecting opportunities of early ART initiation, a strategy that will likely not be applicable to the majority of ongoing infections worldwide [3,4,5].
Consequences of HIV-1 infection that impact non-AIDS aspects of health are an ongoing challenge in clinical care. A vast range of neurological complications, collectively termed HIV-associated neurocognitive disorders (HANDs), have been reported in HIV-1-infected individuals. Even with ART, HAND is associated with greater risk for disease progression and poorer morbidity [6]. It is estimated that up to 50% of ART-treated children will develop neurologic complications [7,8,9,10,11]. Clinical manifestations include mild to severe neurocognitive impairment, delays in motor development, and behavioral psychiatric conditions such as depression and attention-deficit hyperactivity disorder (ADHD). Progressive encephalopathy, described to compromise brain growth, is a prominent and severe presentation in untreated pediatric infection [12]. In fact, the incidence of encephalopathy is higher in infants than adults in the first year of infection, which may be reflective of pathologic events during fetal and early postnatal brain development [13]. While early diagnosis and ART initiation halts and partially reverses progression, static encephalopathy can persist after treatment [14].
The direct cause of developmental disorders is unclear but may be due to factors such as poor penetrance of ART to the central nervous system (CNS) leading to continuous unchecked viral replication in the brain, chronic neuroinflammation, or neurotoxic effects of long-term ART treatment. While these factors have been explored in adults, there is a critical need to further understand these aspects of disease in children who are exposed to HIV and ART during periods of rapid brain development and are often infected orally through breastfeeding, an understudied yet relevant and dominant mucosal transmission route in pediatric infection [15]. Animal models are an important means to address these questions, with the advantage of overcoming challenges faced when using human samples such as limited access to anatomical sites, small sample volumes, and lack of control over experimental variables (i.e., viral dose, transmission route, and time of ART initiation and duration).
Nonhuman primate (NHP) models of HIV/AIDS have long been a powerful platform that have advanced our understanding of HIV transmission, pathogenesis, and persistence. While investigations of pediatric HIV-1 CNS infection using this animal model are limited, findings from these studies are uncovering key differences from adults in neuropathogenesis that could inform advancements in pediatric care (summarized in Table 1). The purpose of this review is to provide insights recently gained from NHP models of pediatric infection of the CNS as well as discuss their implications for the future development of therapies and cure strategies for children living with HIV-1.
SIVs and SHIVs in HIV Research
Asian NHPs, namely rhesus (Macaca mulatta), pigtailed (Macaca nemestrina), and cynomolgus (Macaca fascicaluris) macaques, have become the most commonly used and widely accepted animal models for HIV-1 infection [16,17,18]. Additionally, neurodevelopment is similar between infant humans and macaques, making them suitable for studies of neurologic disease [19,20,21]. With some variability between species, most Asian macaques are readily infected with simian immunodeficiency virus (SIV) and model key viral and immune features of infection such as gradual CD4+ T cell depletion, progression to AIDS, suppression of viremia with ART, and effective transmission through mucosal routes [22]. The low prevalence of CNS disease with the most commonly used strains of SIV can present a challenge for neuropathogenesis studies in macaques, however. As such, SIV and HIV neurotropic strains have been developed and optimized in macaque species to yield more consistent outcomes of CNS disease (Table 2; further described in “NHP Models of Accelerated CNS Disease”).
Although SIV-macaque models have been widely used for studies of HIV-1 transmission, immunopathogenesis, vaccination, and cure, differences in SIV and HIV-1 can make it difficult to address certain experimental questions. For instance, the efficacy of vaccines or entry inhibitors developed against the HIV-1 envelope, a site of heavy divergence between SIV and HIV-1, cannot be directly tested using an SIV challenge. Simian/human immunodeficiency viruses (SHIVs) expressing HIV-1 Env glycoproteins or proteins targeted by antiretrovirals have been constructed to address this gap in translational studies. While initial chimeric variants showed poor replication in macaques, the pathogenicity of next-generation SHIVs has been improved by serial-passage and enhanced affinity for macaque entry receptors [23, 24•, 25, 26]. Studies demonstrating neuroinvasion of SHIV variants in the pediatric NHP setting are limited. The value of these viruses in pre-clinical studies warrants their further characterization and development for investigations of CNS infection.
It is important to consider that studies to date, and described in this review, encompass pediatric NHP models using a range of species, age at challenge, route of infection, virus, and dose (Tables 1 and 2). Thus, it is key to balance reported findings with the suitability of the model used to address the aspect of CNS infection under investigation (i.e., neuroinvasion, target cells of infection, reservoirs, neurological symptoms, etc.).
CNS Entry and Localization
Timing
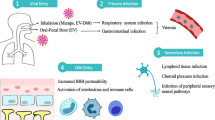
Studies in adult macaques have yielded conflicting models of timing for systemic dissemination after mucosal challenge. While studies of SIV vaginal transmission have reported viral production confined at the port of entry for days before spread, others using the same model have detected virus in draining lymph nodes within 24 h [27,28,29]. Evidence from orally infected infant rhesus macaques shows rapid dissemination of virus to proximal tissues, with viral RNA found in the periphery by 2 days post-challenge [30•, 31]. Thus, after infection across the oral mucosa, the virus quickly gains access to draining lymph nodes of the head and neck which could mediate early invasion of other anatomical sites [30•, 31].
While previous reports have shown SIV in neonatal macaque brains within 3 to 7 days of in utero or i.v. challenge, recent studies of oral transmission have demonstrated even earlier infiltration of this site. In a study of 15 infant macaques infected with SIVmac251 between 3 to 8 weeks of age, SIV DNA was detectable in the brain as early as 48 h after challenge [32•]. 40% of infants analyzed at 48 h had detectable SIV DNA in the cerebrum, with the percentage rising to 67% by 72 h post-challenge. Similar kinetics of viral DNA distribution in infant macaques have also been reported following challenge with SHIVSF162P3, with viral DNA detectable in the cerebellum within 1 day of challenge [30•]. Both studies also measured RNA levels to assess if DNA-positive tissues were sites of productive infection. In the SIVmac251-infected macaques, viral RNA was only detected in 1/15 macaque brains and no RNA was detected in the CSF within 96 h. SHIVSF162P3 RNA was only found in the cerebellum at 14 days but not at 1 day post-challenge. Undetectable levels of RNA in the CNS of these animals before 72 h post-infection may be reflective of recent immigration of infected cells to this site, before localized replication and spread.
Understanding how and when the virus disseminates into the CNS in pediatric HIV-1 infection could reveal how long the window of opportunity is to impede neuroinvasion. The use of oral infection in these described infant macaque studies makes the findings of particular relevance for breastfeeding transmission, the route by which the majority of new pediatric HIV-1 infections are now acquired.
Mechanisms of Entry
The most widely accepted and supported mechanism of CNS entry by HIV/SIV is thought to be chemokine-mediated migration of virally-infected lymphocytes and monocytes across the blood-brain barrier (BBB), where they can release virus to resident target cells [33, 34]. Although not as well-characterized, other mechanisms have been proposed, including entry of cell-free virus through a disrupted BBB or direct infection of cells that line the BBB [35].
Studies of viral CNS entry processes in perinatal infection are limited but suggest that invasion events may differ by developmental stage. In infants and neonates, developing cerebral vessels are more susceptible to damage from drugs, toxins, or neuroinflammation, which could lead to barrier dysfunction [36]. Such damage could provide an opportunity for CNS invasion by free virus. Delery et al. recently demonstrated that the BBB of neonatal rhesus macaques actually remains fairly impermeable during SIV infection [37••]. This would support the role for entry mediated by a “Trojan Horse” or infection of BBB-lining cells. In line with this hypothesis is the previous identification of SIV-infected cells localized to blood vessels in the brains of neonatal macaques infected intravenously [38]. Interestingly, virus was rarely found in these areas in fetal macaques infected in utero [39]. Taken together, these studies provide preliminary evidence for the notion that invasion events could differ by developmental stage at time of infection or transmission route. It is worth noting a limitation of each of these studies was the use of a single technique to draw conclusions on mechanisms of viral invasion. There is more to be learned from carefully designed studies that utilize a combination of ISH, permeability markers, and cell tracking to delineate the major mechanism(s) of viral entry into the neonatal brain with the long-term goal of identifying targets for pre- or post-exposure prophylaxis.
Sites of Infection
In infant macaques, the prevalence of CNS infection is similar to that of juveniles and adults, but differences in the distribution of virally infected cells have been reported. In fetal rhesus macaques infected in utero with SIVmac251, virus-positive cells—identified by DNA, RNA, and protein—were present within the meninges, basal ganglia, stroma of choroid plexus, external granular layer of the cerebellum, cortical plate, and cortical white matter within 15 days post-infection [39]. Of these locations, virus was most frequently found in the cortical white matter. While SIV can be found across this region in juvenile and adult macaques, this is typically only under conditions of encephalitis [40, 41], that was not seen in the infants. An additional study, in which newborn macaques were infected i.v. with SIVmac251, SIVmac239, or SIVmac239/316, also found detectable SIV DNA across multiple overlapping brain regions [38]. Here, infected cells were frequently identified in the cortical gray matter, an area less dominated by SIV infection in older animals [40, 42].
Altered viral distribution in fetal, neonatal, and juvenile infection may reflect expansion in cell tropism at early developmental stages. During gestation, glial and neuronal cells are mitotically-active, which contrasts the more static nature of the adult CNS [43]. Brain regions of ongoing cell proliferation in the fetus, which would be rarer in healthy adults, could then become a unique site of viral replication. Yet, the question remains of how such cell types, like astrocytes, could be targets of infection if they have little to no expression of required entry receptors. A recent report showed a paucity of CCR5+ cells within the brain of uninfected neonatal macaques, despite SIV-infected neonates having similar viral DNA and RNA levels in the brain compared to adults [37••]. This suggests the possibility of alternative means for viral spread in a setting of limited CCR5 availability, such as through the formation of virologic synapses, which could favor infection of cells that would otherwise be spared from direct receptor-mediated infection [44]. Whether astrocytes can support productive infection or reservoir establishment in vivo is still debated, but the presence of virus or viral products in these and other cells, such as microglia and perivascular macrophages, could contribute to bursts of viral release or inflammation from persistent antigen exposure [45,46,47,48]. Extending studies to identify anatomical foci and target cells of HIV/SIV perinatal infection could guide targeted delivery of therapeutics into these regions of early viral replication.
Neuropathogenesis
Histopathological Findings
Differences in virus localization throughout the brain in pediatric infection, as described in the previous section, raises the possibility of altered or accelerated pathogenesis of neurologic disease induced by HIV/SIV in this age group. Previous histological findings in rhesus macaques infected with SIV in utero or shortly after birth show brain pathologies that closely resemble those seen in HIV-1-infected children [49, 50]. Decreased brain growth, evident after 2 months of infection, has been reported in SIV-infected neonatal macaques [38]. Perivascular infiltrates of mononuclear cells, mineralization of vessels in the basal ganglia, and proliferation of glial cells could also be seen within 3 weeks of infection [38, 39]. Although such pathologies were generally associated with regions where virus was detected, it is unclear whether lesions or delayed brain growth are the result of direct or indirect effects of the infection.
A growing body of work is providing anatomical evidence for neurological impairments and disease observed in pediatric HIV-1 infection. Newborn rhesus macaques infected i.v. with SIVmac251 have presented with dramatic reductions in immature neurons and the pyramidal neuron population within the hippocampus at 3 months post-infection [51]. A follow-up study by Carryl et al. reported more pronounced pathological findings when animals were infected orally, although orally infected animals were also older [52••]. Reductions in hippocampal myelination were also evident [53]. Loss of hippocampal neuronal cell types and demyelination could explain the mechanisms underlying the rapid neurocognitive and neuromotor decline sometimes seen in pediatric HIV-1 patients, including deficits in memory and the onset of multiple sclerosis-like illness [54, 55]. Congruency with clinical findings further validates the use of NHP models to evaluate and improve the course of HIV-1 CNS infection in children.
Encephalitis
Despite the presence of virus in the CNS and the incidence of neurologic complications in pediatric HIV-1 infection, reports of encephalitis are scarce [56,57,58]. SIV-infected infant macaques also rarely present with multi-nucleated giant cells in the brain, one histological hallmark of encephalitis [39, 59]. These findings are particularly surprising when one considers the context of perinatal infection, characterized by high plasma viral loads and rapid disease progression. Seeking to address this paradox, a recent retrospective analysis of over 100 SIV-infected rhesus macaques uncovered that incidence of encephalitis is age-dependent [37••]. In this study, no signs of encephalitis were seen in any of the animals infected as neonates (n = 51), with the earliest case observed in an animal infected at 4 months of age. Incidence in juveniles and adults, however, reached approximately 25%. While more direct investigations are needed to uncover features that influence encephalitis susceptibility, these findings highlight the importance of age-spectrum studies which could uncover not only mechanisms underlying accelerated disease progression but also features of protection from HIV-associated pathologies.
NHP Models of Accelerated CNS Disease
Previous studies have shown that about 20–36% of SIVmac-infected rhesus macaques exhibit neuropathological lesions and symptoms of CNS disease, a frequency similar to HIV-1-infected patients [60]. Thus, while SIVmac infection of rhesus macaques provides a strong homolog to HIV-1 infection of humans, the infrequency of neuropathology makes it challenging to use this system for deep investigations of CNS infection, such as uncovering the cause(s) of neuronal dysfunction or loss and their impact on behavior and cognitive abilities. Animal models of accelerated and consistent CNS disease could allow studies of shorter duration with fewer animals to interrogate these processes.
Zink et al. developed such a system by co-inoculating pigtailed macaques with two SIV strains: neurovirulent SIV/17E-Fr and immunosuppressive SIV/DeltaB670 [61]. Over 90% of infected animals developed CSF viral loads on the order of 106 copies/ml by 10 days post-inoculation. Within 3 months, animals progressed to AIDS, developed encephalitis, and displayed neuronal damage. Importantly, a significantly lower prevalence of encephalitis was seen in rhesus or cynomolgus macaques given the same co-inoculation, suggesting that host genetic factors also play a role in neurological disease outcome in this model [61, 62]. It could be of great value to apply this system to fetal or neonatal pigtailed macaques to assess disease events and their impact on neurodevelopment, as has been done previously with HIV-2287 [63, 64]. In addition to high viral loads and neurological lesions, animals in these studies infected in utero or at 1 month of age showed delays in motor and cognitive development. While these NHP models of rapid CNS disease progression may not wholly reflect immune and viral events seen in the slower progression of HIV-1 infection, such models could still deepen our understanding of the sequence of neuropathologic events as well as provide a platform for testing drug candidates that can improve or preserve neurologic functions in pediatric HIV-1 infection.
The CNS as a Latent Reservoir
Viral Persistence on ART
The BBB exists to tightly regulate the entry of solutes and inhibit invasion of pathogens into the CNS; however, it is this feature that contributes to low ART drug penetrance as well as limited immunosurveillance in the CNS [65, 66]. Such circumstances could provide a sanctuary for virally-infected cells and permit ongoing replication. Indeed, untreated and ART-suppressed macaques have comparable frequencies of cells harboring SIVmac251 RNA or DNA in brain tissue [24•, 67, 68]. Recently, our laboratory reported similar findings comparing viremic and ART-suppressed orally-infected infant rhesus macaques [69••]. In addition, we also observed low to undetectable ART drug levels across the brain in all animals, including the cortices, frontal lobe, and basal ganglia. While these observations clearly demonstrate poor clearance of SIV in the brain, whether the CNS could serve as a functional latent reservoir has long been a source of controversy.
Findings from HIV-1-Infected Patients
A growing body of evidence supports the CNS as an anatomical reservoir in HIV-1 infection. HIV-1 RNA has been detected in the CSF but not in the blood of patients on ART [70,71,72,73,74]. This discordance in CSF and plasma viral loads, termed CSF viral escape, raises the possibility of ongoing low-level replication or intermittent bursts of virus production in the CNS even in the absence of systemic HIV replication [75]. CSF viral escape is more prevalent in adults with neurologic symptoms or poorer neurocognitive performance, as is higher levels of persistent HIV DNA in the CSF of adults with viremia suppressed by ART [70, 72, 76, 77•]. Deep-sequencing analysis has revealed compartmentalized viral evolution within the CSF, evidenced by viral populations genetically distinct from those in the blood and capable of contributing as an independent source of viral rebound within the CSF after ART interruption [78,79,80,81,82]. Extensive analyses in this area are generally lacking for perinatal infection. One study has documented CSF compartmentalization by 3 years of age in up to 50% of ART-naïve children infected with HIV-1 subtype C [83]. Here, independent replication in the CNS was proposed to occur by early sequestration of a single transmitted variant to the CNS or by emergence of CNS-adapted variants in later stages of infection.
Macrophages and Microglia as Viral Reservoirs
Resting memory CD4+ T cells are thought to be the predominant source of replication-competent reservoirs in blood and peripheral tissues. However, the genome of rebounding virus cannot always be phylogenetically traced back to proviral genomes in resting CD4+ T cells, indicating the possible existence of a non-CD4+ T cell pool of persistent virus [84,85,86]. Viral DNA and RNA have been found in brain macrophages and resident microglia of SIV-infected infant and adult rhesus macaques as well as in HIV-infected patients [31, 38, 40, 45, 69••, 87,88,89]. Adapting the quantitative viral outgrowth assay (QVOA) to brain macrophages, Avalos et al. showed these cell types harbor replication-competent virus in ART-suppressed pigtailed macaques [90••]. In this same NHP model, treatment with latency reversing agents in vivo led to focal reactivation of viral reservoirs in brain macrophages that, in some animals, occurred independently from the periphery [91]. Experimental CD4 depletion in SIV-infected rhesus macaques has been shown to result in productive infection of macrophages and microglia, with peripheral set point viral loads reaching levels two logs higher than undepleted controls [88, 92]. Taken together, these studies demonstrate myeloid cells in the brain can be targets of SIV infection and harbor replication-competent virus even in the setting of long-term ART treatment.
Advancements in ART delivery to the CNS will likely be insufficient for eradication in myeloid cell types, as many ART drugs already used in the clinic show limited efficacy in microglia and macrophages [93]. The myeloid lineage also presents a particular challenge for cure strategies as they can be long-lived, are capable of self-renewal, and are not efficiently killed by CD8+ T cells [94,95,96]. Thus, efforts for viral clearance, like shock and kill strategies, should also be evaluated for activity against myeloid cells and confirmed in infant models of HIV-1 infection.
Conclusions
NHP models have provided valuable insights into HIV-1 CNS infection, including timing of neuroinvasion, anatomical links to specific neurologic impairments, and identification of cell types harboring latent virus (see Table 1 and Fig. 1). However, there is much to be learned in these areas for perinatally-infected children. While findings in adult humans and NHPs can pave the way for progress in the treatment of CNS disease, it is still critical these studies be validated in pediatric models. Immune and virologic features unique to pediatric infection could impact mechanisms that promote persistence or disease [97•]. For instance, our lab has shown naive CD4+ T cells are the major contributor to the total CD4+ T cell reservoir in SIV-infected infant rhesus macaques, in contrast to central memory CD4+ T cells in adult macaques [69••]. In addition, infant rhesus macaques have a higher baseline turnover rate of monocytes, which further increases during SIV infection and is associated with rapid progression to AIDS [98]. Such findings highlight the necessity for pediatric-focused studies to ensure cure strategies and treatments for neurological impairments will be relevant in this age group.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
https://www.unaids.org/en/resources/fact-sheet. Accessed 11/10/2019.
Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: a collaborative re-analysis. Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action. Concerted Action on SeroConversion to AIDS and Death in Europe. Lancet. 2000;355(9210):1131–7.
Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M Jr, Chun TW, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369(19):1828–35. https://doi.org/10.1056/NEJMoa1302976.
Frange P, Faye A, Avettand-Fenoel V, Bellaton E, Descamps D, Angin M, et al. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV. 2016;3(1):e49–54. https://doi.org/10.1016/S2352-3018(15)00232-5.
Violari A, Cotton MF, Kuhn L, Schramm DB, Paximadis M, Loubser S, et al. A child with perinatal HIV infection and long-term sustained virological control following antiretroviral treatment cessation. Nat Commun. 2019;10(1):412. https://doi.org/10.1038/s41467-019-08311-0.
Pearson DA, NM MG, Nozyce M, Nichols SL, Raskino C, Brouwers P, et al. Predicting HIV disease progression in children using measures of neuropsychological and neurological functioning. Pediatric AIDS clinical trials 152 study team. Pediatrics. 2000;106(6):E76. https://doi.org/10.1542/peds.106.6.e76.
Abubakar A, Van Baar A, Van de Vijver FJ, Holding P, Newton CR. Paediatric HIV and neurodevelopment in sub-Saharan Africa: a systematic review. Tropical medicine & international health : TM & IH. 2008;13(7):880–7. https://doi.org/10.1111/j.1365-3156.2008.02079.x.
Nachman SA, Chernoff M, Gona P, Van Dyke RB, Dankner WM, Seage GR 3rd, et al. Incidence of noninfectious conditions in perinatally HIV-infected children and adolescents in the HAART era. Archives of pediatrics & adolescent medicine. 2009;163(2):164–71. https://doi.org/10.1001/archpedi.163.2.164.
Donald KA, Hoare J, Eley B, Wilmshurst JM. Neurologic complications of pediatric human immunodeficiency virus: implications for clinical practice and management challenges in the African setting. Semin Pediatr Neurol. 2014;21(1):3–11. https://doi.org/10.1016/j.spen.2014.01.004.
Wilmshurst JM, Donald KA, Eley B. Update on the key developments of the neurologic complications in children infected with HIV. Curr Opin HIV AIDS. 2014;9(6):533–8. https://doi.org/10.1097/coh.0000000000000101.
Thakur KT, Boubour A, Saylor D, Das M, Bearden DR, Birbeck GL. Global HIV neurology: a comprehensive review. AIDS (London, England). 2019;33(2):163–84. https://doi.org/10.1097/qad.0000000000001796.
Mirani G, Williams PL, Chernoff M, Abzug MJ, Levin MJ, Seage GR III, et al. Changing trends in complications and mortality rates among US youth and young adults with HIV infection in the era of combination antiretroviral therapy. Clin Infect Dis. 2015;61(12):1850–61. https://doi.org/10.1093/cid/civ687.
Tardieu M, Le Chenadec J, Persoz A, Meyer L, Blanche S, Mayaux MJ. HIV-1–related encephalopathy in infants compared with children and adults. Neurology. 2000;54(5):1089–95. https://doi.org/10.1212/WNL.54.5.1089.
Prato M, Venturini E, Chiappini E, de Martino M, Galli L. Starting treatment in pediatric HIV infection: try to clarify a gray area. Pediatr Infect Dis J. 2015;34(5 Suppl 1):S31–5. https://doi.org/10.1097/inf.0000000000000662.
Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13(11):976–86. https://doi.org/10.1016/S1473-3099(13)70269-X.
Hatziioannou T, Evans DT. Animal models for HIV/AIDS research. Nat Rev Microbiol. 2012;10(12):852–67. https://doi.org/10.1038/nrmicro2911.
Kumar N, Chahroudi A, Silvestri G. Animal models to achieve an HIV cure. Curr Opin HIV AIDS. 2016;11(4):432–41. https://doi.org/10.1097/COH.0000000000000290.
Nixon CC, Mavigner M, Silvestri G, Garcia JV. In Vivo Models of Human Immunodeficiency Virus Persistence and Cure Strategies. J Infect Dis. 2017;215(suppl_3):S142–S51. https://doi.org/10.1093/infdis/jiw637.
Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN. The prefrontal cortex: comparative architectonic organization in the human and the macaque monkey brains. Cortex. 2012;48(1):46–57. https://doi.org/10.1016/j.cortex.2011.07.002.
Nowakowski RS, Rakic P. The site of origin and route and rate of migration of neurons to the hippocampal region of the rhesus monkey. J Comp Neurol. 1981;196(1):129–54. https://doi.org/10.1002/cne.901960110.
Rakic P, Nowakowski RS. The time of origin of neurons in the hippocampal region of the rhesus monkey. J Comp Neurol. 1981;196(1):99–128. https://doi.org/10.1002/cne.901960109.
Veazey RS, Lackner AA. Nonhuman primate models and understanding the pathogenesis of HIV infection and AIDS. ILAR J. 2017;58(2):160–71. https://doi.org/10.1093/ilar/ilx032.
Reimann KA, Li JT, Veazey R, Halloran M, Park IW, Karlsson GB, et al. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70(10):6922–8.
• Estes JD, Kityo C, Ssali F, Swainson L, Makamdop KN, Del Prete GQ, et al. Defining total-body AIDS-virus burden with implications for curative strategies. Nature medicine. 2017;23(11):1271–6. https://doi.org/10.1038/nm.4411In both SIV and SHIV infections, the level of viral RNA+ cells in the brain was shown to be similar in ART-treated and untreated adult macaques.
Bar KJ, Coronado E, Hensley McBain T, O'Connor MA, Osborn JM, Miller C, et al. Simian-Human Immunodeficiency Virus SHIV.CH505 Infection of Rhesus Macaques Results in Persistent Viral Replication and Induces Intestinal Immunopathology. J Virol. 2019;93(18). https://doi.org/10.1128/jvi.00372-19.
Li H, Wang S, Kong R, Ding W, Lee FH, Parker Z, et al. Envelope residue 375 substitutions in simian-human immunodeficiency viruses enhance CD4 binding and replication in rhesus macaques. Proc Natl Acad Sci U S A. 2016;113(24):E3413–22. https://doi.org/10.1073/pnas.1606636113.
Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74(13):6087–95. https://doi.org/10.1128/jvi.74.13.6087-6095.2000.
Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science (New York, NY). 1999;286(5443):1353–7. https://doi.org/10.1126/science.286.5443.1353.
Deleage C, Immonen TT, Fennessey CM, Reynaldi A, Reid C, Newman L, et al. Defining early SIV replication and dissemination dynamics following vaginal transmission. Science Advances. 2019;5(5):eaav7116. https://doi.org/10.1126/sciadv.aav7116.
• Hessell AJ, Jaworski JP, Epson E, Matsuda K, Pandey S, Kahl C, et al. Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nature Medicine. 2016;22(4):362–8. https://doi.org/10.1038/nm.4063This study shows rapid dissemination of SHIV following oral challenge in neonatal rhesus macaques.
Milush JM, Kosub D, Marthas M, Schmidt K, Scott F, Wozniakowski A et al. Rapid dissemination of SIV following oral inoculation. AIDS (London, England). 2004;18(18):2371–80.
• Amedee AM, Phillips B, Jensen K, Robichaux S, Lacour N, Burke M, et al. Early sites of virus replication after oral SIVmac251 infection of infant macaques: implications for pathogenesis. AIDS Research and Human Retroviruses. 2018;34(3):286–99. https://doi.org/10.1089/aid.2017.0169This work describes early dissemination events after oral challenge of infant macaques.
Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood–brain barrier: a potential mechanism of HIV–CNS invasion and NeuroAIDS. J Neurosci. 2006;26(4):1098–106. https://doi.org/10.1523/JNEUROSCI.3863-05.2006.
Zink MC, Coleman GD, Mankowski JL, Adams RJ, Tarwater PM, Fox K, et al. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J Infect Dis. 2001;184(8):1015–21. https://doi.org/10.1086/323478.
Atluri VSR, Hidalgo M, Samikkannu T, Kurapati KRV, Jayant RD, Sagar V, et al. Effect of human immunodeficiency virus on blood-brain barrier integrity and function: an update. Front Cell Neurosci. 2015;9:212. https://doi.org/10.3389/fncel.2015.00212.
Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Front Pharmacol. 2012;3:46. https://doi.org/10.3389/fphar.2012.00046.
•• Delery E, Bohannon DG, Irons DL, Allers C, Sugimoto C, Cai Y, et al. Lack of susceptibility in neonatally infected rhesus macaques to simian immunodeficiency virus-induced encephalitis. J Neurovirol. 2019;25(4):578–88. https://doi.org/10.1007/s13365-019-00755-wThese findings propose mechanisms underlying differences in susceptibility to SIV encephalitis across infant, juvenile, and adult rhesus macaques.
Westmoreland SV, Williams KC, Simon MA, Bahn ME, Rullkoetter AE, Elliott MW, et al. Neuropathogenesis of simian immunodeficiency virus in neonatal rhesus macaques. Am J Pathol. 1999;155(4):1217–28. https://doi.org/10.1016/s0002-9440(10)65224-8.
Lane JH, Tarantal AF, Pauley D, Marthas M, Miller CJ, Lackner AA. Localization of simian immunodeficiency virus nucleic acid and antigen in brains of fetal macaques inoculated in utero. Am J Pathol. 1996;149(4):1097–104.
Lackner AA, Smith MO, Munn RJ, Martfeld DJ, Gardner MB, Marx PA, et al. Localization of simian immunodeficiency virus in the central nervous system of rhesus monkeys. Am J Pathol. 1991;139(3):609–21.
Xing HQ, Mori K, Sugimoto C, Ono F, Izumo K, Kuboda R, et al. Impaired astrocytes and diffuse activation of microglia in the cerebral cortex in simian immunodeficiency virus-infected macaques without simian immunodeficiency virus encephalitis. J Neuropathol Exp Neurol. 2008;67(6):600–11. https://doi.org/10.1097/NEN.0b013e3181772ce0.
Lackner AA, Vogel P, Ramos RA, Kluge JD, Marthas M. Early events in tissues during infection with pathogenic (SIVmac239) and nonpathogenic (SIVmac1A11) molecular clones of simian immunodeficiency virus. Am J Pathol. 1994;145(2):428–39.
Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20(4):327–48. https://doi.org/10.1007/s11065-010-9148-4.
Do T, Murphy G, Earl LA, Del Prete GQ, Grandinetti G, Li GH, et al. Three-dimensional imaging of HIV-1 virological synapses reveals membrane architectures involved in virus transmission. J Virol. 2014;88(18):10327–39. https://doi.org/10.1128/jvi.00788-14.
Ko A, Kang G, Hattler JB, Galadima HI, Zhang J, Li Q, et al. Macrophages but not astrocytes harbor HIV DNA in the brains of HIV-1-infected aviremic individuals on suppressive antiretroviral therapy. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2019;14(1):110–9. https://doi.org/10.1007/s11481-018-9809-2.
Russell RA, Chojnacki J, Jones DM, Johnson E, Do T, Eggeling C, et al. Astrocytes resist HIV-1 fusion but engulf infected macrophage material. Cell Rep. 2017;18(6):1473–83. https://doi.org/10.1016/j.celrep.2017.01.027.
Chauhan A, Khandkar M. Endocytosis of human immunodeficiency virus 1 (HIV-1) in astrocytes: a fiery path to its destination. Microb Pathog. 2015;78:1–6. https://doi.org/10.1016/j.micpath.2014.11.003.
Al-Harti L, Joseph J, Nath A. Astrocytes as an HIV CNS reservoir: highlights and reflections of an NIMH-sponsored symposium. J Neurovirol. 2018;24(6):665–9. https://doi.org/10.1007/s13365-018-0691-8.
Belman AL, Ultmann MH, Horoupian D, Novick B, Spiro AJ, Rubinstein A, et al. Neurological complications in infants and children with acquired immune deficiency syndrome. Ann Neurol. 1985;18(5):560–6. https://doi.org/10.1002/ana.410180509.
George R, Andronikou S, du Plessis J, du Plessis AM, Van Toorn R, Maydell A. Central nervous system manifestations of HIV infection in children. Pediatr Radiol. 2009;39(6):575–85. https://doi.org/10.1007/s00247-009-1170-4.
Curtis K, Rollins M, Carryl H, Bradshaw K, Van Rompay KK, Abel K, et al. Reduction of pyramidal and immature hippocampal neurons in pediatric simian immunodeficiency virus infection. Neuroreport. 2014;25(13):973–8. https://doi.org/10.1097/wnr.0000000000000148.
•• Carryl H, Van Rompay KK, De Paris K, Burke MW. Hippocampal neuronal loss in infant macaques orally infected with virulent simian immunodeficiency virus (SIV). Brain Sciences. 2017;7(4). https://doi.org/10.3390/brainsci7040040Follow-up to Curtiset al.2014 study describing pathological findings in the hippocampus of SIV-infected infant macaques which could explain neurocognitive impairments in pediatric HIV-1 cases.
Kamboj H, Curtis K, Carryl H, Agyemang H, Van Rompay K, Abel K et al. Central nervous system demyelination in pediatric SIV infection. Conference on Retroviruses and Opportunistic Infections; March 3-6, 2014; Boston, MA2014.
Facchini SA, Harding SA, Waldron RL. Human immunodeficiency virus-1 infection and multiple sclerosis-like illness in a child. Pediatr Neurol. 2002;26(3):231–5. https://doi.org/10.1016/S0887-8994(01)00378-2.
Hoare J, Fouche JP, Spottiswoode B, Donald K, Philipps N, Bezuidenhout H, et al. A diffusion tensor imaging and neurocognitive study of HIV-positive children who are HAART-naive “slow progressors”. J Neuro-Oncol. 2012;18(3):205–12. https://doi.org/10.1007/s13365-012-0099-9.
Kure K, Llena JF, Lyman WD, Soeiro R, Weidenheim KM, Hirano A, et al. Human immunodeficiency virus-1 infection of the nervous system: an autopsy study of 268 adult, pediatric, and fetal brains. Hum Pathol. 1991;22(7):700–10. https://doi.org/10.1016/0046-8177(91)90293-X.
Vazeux R, Lacroix-Ciaudo C, Blanche S, Cumont MC, Henin D, Gray F, et al. Low levels of human immunodeficiency virus replication in the brain tissue of children with severe acquired immunodeficiency syndrome encephalopathy. Am J Pathol. 1992;140(1):137–44.
Lanjewar D, Bhatia V, Lanjewar S. Pathologic lesions in children with acquired immunodeficiency syndrome an autopsy study of 11 cases from Mumbai, India. Indian J Pathol Microbiol. 2016;59(2):166–71. https://doi.org/10.4103/0377-4929.182028.
Colonna L, Peterson CW, Schell JB, Carlson JM, Tkachev V, Brown M, et al. Evidence for persistence of the SHIV reservoir early after MHC haploidentical hematopoietic stem cell transplantation. Nat Commun. 2018;9(1):4438. https://doi.org/10.1038/s41467-018-06736-7.
Williams R, Bokhari S, Silverstein P, Pinson D, Kumar A, Buch S. Nonhuman primate models of NeuroAIDS. J Neurovirol. 2008;14(4):292–300. https://doi.org/10.1080/13550280802074539.
Zink MC, Amedee AM, Mankowski JL, Craig L, Didier P, Carter DL, et al. Pathogenesis of SIV encephalitis. Selection and replication of neurovirulent SIV. Am J Pathol. 1997;151(3):793–803.
Beck SE, Kelly KM, Queen SE, Adams RJ, Zink MC, Tarwater PM, et al. Macaque species susceptibility to simian immunodeficiency virus: increased incidence of SIV central nervous system disease in pigtailed macaques versus rhesus macaques. J Neuro-Oncol. 2015;21(2):148–58. https://doi.org/10.1007/s13365-015-0313-7.
Kinman LM, Worlein JM, Leigh J, Bielefeldt-Ohmann H, Anderson DM, Hu SL, et al. HIV in central nervous system and behavioral development: an HIV-2287 macaque model of AIDS. AIDS (London, England). 2004;18(10):1363–70. https://doi.org/10.1097/01.aids.0000131307.62828.a1.
Worlein JM, Leigh J, Larsen K, Kinman L, Schmidt A, Ochs H, et al. Cognitive and motor deficits associated with HIV-2(287) infection in infant pigtailed macaques: a nonhuman primate model of pediatric neuro-AIDS. J Neuro-Oncol. 2005;11(1):34–45. https://doi.org/10.1080/13550280590901732.
Decloedt EH, Rosenkranz B, Maartens G, Joska J. Central nervous system penetration of antiretroviral drugs: pharmacokinetic, pharmacodynamic and pharmacogenomic considerations. Clin Pharmacokinet. 2015;54(6):581–98. https://doi.org/10.1007/s40262-015-0257-3.
Caniglia EC, Cain LE, Justice A, Tate J, Logan R, Sabin C, et al. Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology. 2014;83(2):134–41. https://doi.org/10.1212/WNL.0000000000000564.
Perez S, Johnson A-M, Xiang S-H, Li J, Foley BT, Doyle-Meyers L, et al. Persistence of SIV in the brain of SIV-infected Chinese rhesus macaques with or without antiretroviral therapy. J Neurovirol. 2018;24(1):62–74. https://doi.org/10.1007/s13365-017-0594-0.
Clements JE, Li M, Gama L, Bullock B, Carruth LM, Mankowski JL, et al. The central nervous system is a viral reservoir in simian immunodeficiency virus--infected macaques on combined antiretroviral therapy: a model for human immunodeficiency virus patients on highly active antiretroviral therapy. J Neuro-Oncol. 2005;11(2):180–9. https://doi.org/10.1080/13550280590922748-1.
•• Mavigner M, Habib J, Deleage C, Rosen E, Mattingly C, Bricker K, et al. Simian Immunodeficiency virus persistence in cellular and anatomic reservoirs in antiretroviral therapy-suppressed infant rhesus macaques. J Virol. 2018;92(18). https://doi.org/10.1128/jvi.00562-18This study uses an infant macaque model of oral SIV infection and long-term ART treatment to uncover anatomic sites of persistence, including the brain.
Canestri A, Lescure F-X, Jaureguiberry S, Moulignier A, Amiel C, Marcelin A, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50(5):773–8. https://doi.org/10.1086/650538.
Eden A, Fuchs D, Hagberg L, Nilsson S, Spudich S, Svennerholm B, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis. 2010;202(12):1819–25. https://doi.org/10.1086/657342.
Peluso MJ, Ferretti F, Peterson J, Lee E, Fuchs D, Boschini A, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS (London, England). 2012;26(14):1765–74. https://doi.org/10.1097/QAD.0b013e328355e6b2.
Ferretti F, Gisslen M, Cinque P, Price RW. Cerebrospinal fluid HIV escape from antiretroviral therapy. Current HIV/AIDS reports. 2015;12(2):280–8. https://doi.org/10.1007/s11904-015-0267-7.
Perez-Valero I, Ellis R, Heaton R, Deutsch R, Franklin D, Clifford DB, et al. Cerebrospinal fluid viral escape in aviremic HIV-infected patients receiving antiretroviral therapy: prevalence, risk factors and neurocognitive effects. AIDS (London, England). 2019;33(3):475–81. https://doi.org/10.1097/qad.0000000000002074.
Joseph SB, Kincer LP, Bowman NM, Evans C, Vinikoor MJ, Lippincott CK, et al. Human immunodeficiency virus type 1 RNA detected in the central nervous system (CNS) after years of suppressive antiretroviral therapy can originate from a replicating CNS reservoir or clonally expanded cells. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2019;69(8):1345–52. https://doi.org/10.1093/cid/ciy1066.
Tiraboschi JM, Munoz-Moreno JA, Puertas MC, Alonso-Villaverde C, Prats A, Ferrer E, et al. Viral and inflammatory markers in cerebrospinal fluid of patients with HIV-1-associated neurocognitive impairment during antiretroviral treatment switch. HIV Medicine. 2015;16(6):388–92. https://doi.org/10.1111/hiv.12243.
• Spudich S, Robertson KR, Bosch RJ, Gandhi RT, Cyktor JC, Mar H, et al. Persistent HIV-infected cells in cerebrospinal fluid are associated with poorer neurocognitive performance. The Journal of Clinical Investigation. 2019;129(8):3339–46. https://doi.org/10.1172/jci127413This study reports 48% of ART-suppressed adult participants have detectable cell-associated HIV-DNA in CSF that is associated with poorer neurocognitive outcomes. Similar data are not yet available for perinatally HIV-infected children.
Schnell G, Joseph S, Spudich S, Price RW, Swanstrom R. HIV-1 replication in the central nervous system occurs in two distinct cell types. PLoS Pathog. 2011;7(10):e1002286. https://doi.org/10.1371/journal.ppat.1002286.
Dahl V, Peterson J, Fuchs D, Gisslen M, Palmer S, Price RW. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS (London, England). 2014;28(15):2251–8. https://doi.org/10.1097/QAD.0000000000000400.
Tong CYW, Costelloe S, Hubb J, Mullen J, O'Shea S, Marta M, et al. Deep sequencing of HIV-1 in cerebrospinal fluid. Clin Infect Dis. 2015;61(6):1022–5. https://doi.org/10.1093/cid/civ417.
Sturdevant CB, Joseph SB, Schnell G, Price RW, Swanstrom R, Spudich S. Compartmentalized replication of R5 T cell-tropic HIV-1 in the central nervous system early in the course of infection. PLoS Pathog. 2015;11(3):e1004720. https://doi.org/10.1371/journal.ppat.1004720.
Gianella S, Kosakovsky Pond SL, Oliveira MF, Scheffler K, Strain MC, De la Torre A et al. Compartmentalized HIV rebound in the central nervous system after interruption of antiretroviral therapy. Virus Evol. 2016;2(2):vew020-vew. doi:https://doi.org/10.1093/ve/vew020.
Sturdevant CB, Dow A, Jabara CB, Joseph SB, Schnell G, Takamune N, et al. Central nervous system compartmentalization of HIV-1 subtype C variants early and late in infection in young children. PLoS Pathog. 2012;8(12):e1003094. https://doi.org/10.1371/journal.ppat.1003094.
Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5(5):512–7. https://doi.org/10.1038/8394.
Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science (New York, NY). 1997;278(5341):1295–300. doi:https://doi.org/10.1126/science.278.5341.1295.
Chun TW, Davey RT Jr, Ostrowski M, Shawn Justement J, Engel D, Mullins JI, et al. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat Med. 2000;6(7):757–61. https://doi.org/10.1038/77481.
Chakrabarti L, Hurtrel M, Maire MA, Vazeux R, Dormont D, Montagnier L, et al. Early viral replication in the brain of SIV-infected rhesus monkeys. Am J Pathol. 1991;139(6):1273–80.
Micci L, Alvarez X, Iriele RI, Ortiz AM, Ryan ES, McGary CS, et al. CD4 depletion in SIV-infected macaques results in macrophage and microglia infection with rapid turnover of infected cells. PLoS Pathog. 2014;10(10):e1004467. https://doi.org/10.1371/journal.ppat.1004467.
Lamers SL, Rose R, Ndhlovu LC, Nolan DJ, Salemi M, Maidji E, et al. The meningeal lymphatic system: a route for HIV brain migration? J Neuro-Oncol. 2016;22(3):275–81. https://doi.org/10.1007/s13365-015-0399-y.
•• Avalos CR, Abreu CM, Queen SE, Li M, Price S, Shirk EN, et al. Brain macrophages in simian immunodeficiency virus-infected, antiretroviral-suppressed macaques: a functional latent reservoir. mBio. 2017;8(4). https://doi.org/10.1128/mBio.01186-17This study applies a novel macrophage QVOA to demonstrate persistent virus in brain macrophages isolated from SIV-infected, ART-suppressed macaques is replication-competent.
Gama L, Abreu CM, Shirk EN, Price SL, Li M, Laird GM, et al. Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques. AIDS (London, England). 2017;31(1):5–14. https://doi.org/10.1097/qad.0000000000001267.
Ortiz AM, Klatt NR, Li B, Yi Y, Tabb B, Hao XP, et al. Depletion of CD4(+) T cells abrogates post-peak decline of viremia in SIV-infected rhesus macaques. J Clin Invest. 2011;121(11):4433–45. https://doi.org/10.1172/jci46023.
Asahchop EL, Meziane O, Mamik MK, Chan WF, Branton WG, Resch L, et al. Reduced antiretroviral drug efficacy and concentration in HIV-infected microglia contributes to viral persistence in brain. Retrovirology. 2017;14(1):47. https://doi.org/10.1186/s12977-017-0370-5.
Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. https://doi.org/10.1016/j.immuni.2013.04.004.
Vojnov L, Martins MA, Bean AT, Veloso de Santana MG, Sacha JB, Wilson NA et al. The majority of freshly sorted simian immunodeficiency virus (SIV)-specific CD8<sup>+</sup> T cells cannot suppress viral replication in SIV-infected macrophages. J Virol 2012;86(8):4682–4687. doi:https://doi.org/10.1128/jvi.06324-11.
Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10(12):1538–43. https://doi.org/10.1038/nn2014.
• Goulder PJ, Lewin SR, Leitman EM. Paediatric HIV Infection: the potential for cure. Nature Reviews Immunology. 2016;16(4):259–71. https://doi.org/10.1038/nri.2016.19This review highlights pediatric factors that could influence reservoir dynamics and cure strategies.
Sugimoto C, Merino KM, Hasegawa A, Wang X, Alvarez XA, Wakao H, et al. Critical role for monocytes/macrophages in rapid progression to AIDS in pediatric simian immunodeficiency virus-infected rhesus macaques. J Virol. 2017;91(17):e00379–17. https://doi.org/10.1128/JVI.00379-17.
Lewis MG, Bellah S, McKinnon K, Yalley-Ogunro J, Zack PM, Elkins WR, et al. Titration and characterization of two rhesus-derived SIVmac challenge stocks. AIDS Res Hum Retrovir. 1994;10(2):213–20. https://doi.org/10.1089/aid.1994.10.213.
Ho RJ, Larsen K, Kinman L, Sherbert C, Wang XY, Finn E, et al. Characterization of a maternal-fetal HIV transmission model using pregnant macaques infected with HIV-2(287). J Med Primatol. 2001;30(3):131–40. https://doi.org/10.1111/j.1600-0684.2001.tb00001.x.
Harouse JM, Gettie A, Eshetu T, Tan RC, Bohm R, Blanchard J, et al. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3). J Virol. 2001;75(4):1990–5. https://doi.org/10.1128/JVI.75.4.1990-1995.2001.
Amedee AM, Lacour N, Gierman JL, Martin LN, Clements JE, Bohm R Jr, et al. Genotypic selection of simian immunodeficiency virus in macaque infants infected transplacentally. J Virol. 1995;69(12):7982–90.
Acknowledgements
AC receives support from the U.S. National Institutes of Health (1P01AI131276, R01A133706).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Central Nervous System and Cognition
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Obregon-Perko, V., Bricker, K. & Chahroudi, A. The Brain Retains: Nonhuman Primate Models for Pediatric HIV-1 in the CNS. Curr HIV/AIDS Rep 17, 343–353 (2020). https://doi.org/10.1007/s11904-020-00503-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-020-00503-4