Abstract
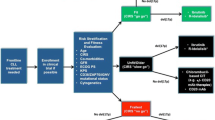
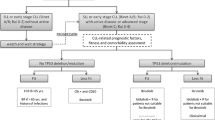
Despite advances in treatment, chronic lymphocytic leukemia (CLL) remains incurable with standard therapies. Novel therapeutic agents are needed, particularly for patients with high-risk cytogenetic abnormalities such as del(17p13). The past year has seen several advances in this field. The immunomodulatory drug lenalidomide and the cyclin-dependent kinase inhibitor flavopiridol demonstrated clinical activity in fludarabine-refractory CLL patients with high-risk cytogenetic features and bulky lymphadenopathy, but they were associated with toxicities such as tumor flare and tumor lysis. Second-generation monoclonal anti-CD20 antibodies in clinical trials include ofatumumab, which demonstrated activity in fludarabine-refractory patients with bulky lymphadenopathy. Oblimersen, obatoclax, and ABT-263 target the antiapoptotic protein Bcl-2. Investigational agents with novel therapeutic targets include the anti-CD37 small modular immunopharmaceutical TRU-016, the oral spleen tyrosine kinase (Syk) inhibitor fostamatinib, and the oral phosphatidylinositol-3-kinase (PI3K) inhibitor CAL-101; all of these have all shown preliminary evidence of clinical activity. The development of novel agents for treating CLL remains an active, exciting area of research.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Chanan-Khan A, Miller KC, Musial L, et al.: Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol 2006, 24:5343–5349.
• Ferrajoli A, Lee BN, Schlette EJ, et al.: Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood 2008, 111:5291–5297 This paper describes a phase 2 study that highlighted the clinical activity of lenalidomide in relapsed CLL, but also the hematologic toxicity and tumor flare reaction observed with its use.
Ferrajoli A, Keating MJ, Wierda WG, et al.: Lenalidomide is active in patients with relapsed/refractory chronic lymphocytic leukemia (CLL) carrying unfavorable chromosomal abnormalities [abstract]. Blood (ASH Annual Meeting Abstracts) 2007, 110:Abstract 754.
Wendtner CM, Mahadevan D, Stilgenbauer S, et al.: Preliminary results of a phase 1/2, multi-center, open-label study (CLL-001) investigating a stepwise dose-escalation schedule of lenalidomide in relapsed or refractory chronic lymphocytic leukemia [abstract]. Blood (ASH Annual Meeting Abstracts) 2008, 112:Abstract 2104.
Andritsos LA, Johnson AJ, Lozanski G, et al.: Higher doses of lenalidomide are associated with unacceptable toxicity including life-threatening tumor flare in patients with chronic lymphocytic leukemia. J Clin Oncol 2008, 26:2519–2525.
Chen C, Paul H, Xu W, et al.: A phase II study of lenalidomide in previously untreated, symptomatic chronic lymphocytic leukemia (CLL) [abstract 44]. Blood 2008, 112:23.
Ferrajoli A, O’Brien S, Wierda W, et al.: Lenalidomide as initial treatment of elderly patients with chronic lymphocytic leukemia (CLL) [abstract]. Blood (ASH Annual Meeting Abstracts) 2008, 112:Abstract 45.
Lin TS, Howard OM, Neuberg DS, et al.: Seventy-two hour continuous infusion flavopiridol in relapsed and refractory mantle cell lymphoma. Leuk Lymphoma 2002, 43:793–797.
Flinn IW, Byrd JC, Bartlett N, et al.: Flavopiridol administered as a 24-hour continuous infusion in chronic lymphocytic leukemia lacks clinical activity. Leuk Res 2005, 29:1253–1257.
Byrd JC, Peterson BL, Gabrilove J, et al.: Treatment of relapsed chronic lymphocytic leukemia by 72-hour continuous infusion or 1-hour bolus infusion of flavopiridol: results from Cancer and Leukemia Group B study 19805. Clin Cancer Res 2005, 11:4176–4181.
Christian BA, Fischer B, Blum KA, et al.: Flavopiridol in chronic lymphocytic leukemia (CLL). Clin Leuk 2007, 1:292–297.
Christian BA, Grever MR, Byrd JC, Lin TS: Flavopiridol in the treatment of chronic lymphocytic leukemia. Curr Opin Oncol 2007, 19:573–578.
Byrd JC, Lin TS, Dalton JT, et al.: Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood 2007, 109:399–404.
Phelps MA, Lin TS, Johnson AJ, et al.: Clinical response and pharmacokinetics from a phase I study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood 2009, 113:2637–2645.
• Lin TS, Ruppert AS, Johnson AJ, et al.: Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol 2009 Oct 13 (Epub ahead of print) This paper summarizes a phase 2 study confirming the clinical activity of flavopiridol in genetically high-risk relapsed CLL and describes modifications to the dosing schedule to reduce cytokine release syndrome symptoms, improve tolerability, and improve treatment delivery.
Lin TS, Heerema NA, Lozanski G, et al.: Flavopiridol (alvocidib) induces durable responses in relapsed chronic lymphocytic leukemia (CLL) patients with high-risk cytogenetic abnormalities [abstract]. Blood (ASH Annual Meeting Abstracts) 2008, 112:Abstract 46.
Lin TS, Blum KA, Fischer B, et al.: Flavopiridol, fludarabine and rituximab (FFR) is an active regimen in mantle cell lymphoma, indolent B-cell lymphomas and chronic lymphocytic leukemia. J Clin Oncol 2010 (in press).
Christian BA, Lin TS: Antibody therapy for CLL. Semin Hematol 2008, 45:95–103.
Teeling JL, French RR, Cragg MS, et al.: Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood 2004, 104:1793–1800.
• Coiffier B, Lepretre S, Pedersen LM, et al.: Safety and efficacy of ofatumumab, a fully humanized monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: A phase 1-2 study. Blood 2008, 111:1094–1100 This paper describes the first clinical study of ofatumumab in relapsed CLL and demonstrates the improved clinical activity of this novel anti-CD20 antibody in CLL.
Osterborg A, Kipps TJ, Mayer J, et al.: Ofatumumab (HuMax-CD20), a novel CD20 monoclonal antibody, is an active treatment for patients with CLL refractory to both fludarabine and alemtuzumab or bulky fludarabine-refractory disease: results from the planned interim analysis of an international pivotal trial [abstract]. Blood (ASH Annual Meeting Abstracts) 2008, 112:Abstract 328.
Allen SL, Rai KR, Elstrom R, et al.: Subcutaneous injections of low doses of veltuzumab (humanized anti-CD20 antibody): objective responses in B-cell malignancies. J Clin Oncol 2009, 27 (suppl):Abstract 8530.
O’Brien S, Cunningham CC, Golenkov AK, et al.: Phase I to II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in patients with advanced chronic lymphocytic leukemia. J Clin Oncol 2005, 23:7697–7702.
O’Brien S, Moore JO, Boyd TE, et al.: Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol 2007, 25:1114–1120.
O’Brien S, Wu J, Novick SC, Rai KR: 5-year follow-up of patients with relapsed/refractory CLL treated with standard chemotherapy with or without oblimersen in randomized phase III trial: prognostic factors and predictive factors for treatment effect [abstract]. Blood (ASH Annual Meeting Abstracts) 2008, 112:Abstract 4201.
O’Brien SM, Claxton DF, Crump M, et al.: Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood 2009, 113:299–305.
• Tse C, Shoemaker AR, Adickes J, et al.: ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 2008, 68:3421–3428 This paper summarizes the preclinical studies demonstrating the biologic activity of the Bcl-2 inhibitor ABT-263, which has shown clinical activity in initial phase 1/2 studies.
Wilson WH, O’Connor OA, Roberts AW, et al.: ABT-263 activity and safety in patients with relapsed or refractory lymphoid malignancies in particular chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) [abstract]. J Clin Oncol 2009, 27(suppl):Abstract 8574.
Andritsos LA, Furman RR, Flinn IW, et al.: A phase I trial of TRU-016, an anti-CD37 small modular immunopharmaceutical (SMIP) in relapsed and refractory CLL [abstract]. J Clin Oncol 2009, 27(suppl):Abstract 3017.
Friedberg JW, Sharman J, Schaefer-Cutillo J, et al.: Fostamatinib disodium (FosD), an oral inhibitor of Syk, is well-tolerated and has significant clinical activity in diffuse large B cell lymphoma (DLBCL) and chronic lymphocytic leukemia (SLL/CLL) [abstract]. Blood (ASH Annual Meeting Abstracts) 2008, 112:Abstract 3.
May SE, Kashishian A, Lin TS, et al.: CAL-101, a selective inhibitor of the p110d isoform of phosphatidylinositol 3-kinase, effectively induces apoptosis in primary chronic lymphocytic leukemia cells providing a novel therapeutic strategy for the treatment of this disease [abstract]. Blood (ASH Annual Meeting Abstracts) 2008, 112:Abstract 3165.
Flinn IW, Byrd JC, Furman RR, et al.: Preliminary evidence of clinical activity in a phase I study of CAL-101, a selective inhibitor of the p110d isoform of phosphatidylinositol 3-kinase (PI3K), in patients with select hematologic malignancies. J Clin Oncol 2009, 27 (suppl):Abstract 3543.
Disclosure
The author wrote this article while a faculty member at The Ohio State University, but he is currently employed by GlaxoSmithKline (Collegeville, PA), co-developer and co-marketer of ofatumumab (Arzerra). He was formerly a consultant and member of Advisory Boards for Genentech and received research funding from Sanofi-Aventis. No other potential conflict of interest relevant to this article was reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, T.S. New Agents in Chronic Lymphocytic Leukemia. Curr Hematol Malig Rep 5, 29–34 (2010). https://doi.org/10.1007/s11899-009-0039-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-009-0039-9