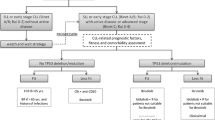
Opinion statement
The better understanding of the biology of chronic lymphocytic leukemia (CLL) gained over the past decade has led to the development and introduction of several targeted drugs, with an demonstrable improvement in the prognosis for this currently incurable condition. Currently, Bruton’s tyrosine kinase (BTK) inhibitors, phosphoinositide 3-kinase (PI3K) inhibitors, venetoclax, and CD20 monoclonal antibodies are the key elements in the treatment of both previously untreated and relapsed/refractory CLL patients. Ibrutinib was the first BTK inhibitor approved for clinical use, and showed excellent efficacy and an acceptable safety profile. Following this, the better-tolerated second-generation irreversible BTK inhibitors acalabrutinib and zanubrutinib have been introduced for the treatment of lymphoid malignancies, and acalabrutinib was approved for CLL. When used as single drugs, BTK inhibitors are given continuously until unacceptable toxicity or disease progression; however, when combined with venetoclax and/or CD20 antibodies, they induce deeper response and can be given for a limited time. Recently, promising new reversible BTK inhibitors pirtobrutinib and nemtabrutinib were discovered, and these seem to be more active and better tolerated than their irreversible predecessors. However, they are in an early phase of development and are not currently approved for CLL. The phosphatidylinositol 3-kinase (PI3K) inhibitors idelalisib and duvelisib are highly effective in patients with relapsed CLL, including high-risk disease. The major limitations for their use are adverse events, mostly of autoimmune origin (hepatitis, enteritis/colitis, and pneumonitis). Otherwise, cellular therapies like allogeneic hematopoietic stem cell transplantation and chimeric antigen receptor (CAR) T cells and bispecific monoclonal antibodies offer promise for patients who have failed BTK inhibitors and venetoclax treatment. In the coming years, it is likely that novel targeted therapies will replace immunochemotherapy regimens in most patients.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hallek M, Al-Sawaf O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol. 2021;96(12):1679–705. https://doi.org/10.1002/ajh.26367.
Eichhorst B, Robak T, Montserrat E, Ghia P, Niemann CU, Kater AP, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:23–33. https://doi.org/10.1016/j.annonc.2020.09.019.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. https://doi.org/10.3322/caac.21708.
Dighiero G, Maloum K, Desablens B, Cazin B, Navarro M, Leblay R, et al. French Cooperative Group on Chronic Lymphocytic Leukemia. Chlorambucil in indolent chronic lymphocytic leukemia. N Engl J Med. 1998;338(21):1506–14. https://doi.org/10.1056/NEJM199805213382104.
Herling CD, Cymbalista F, Groß-Ophoff-Müller C, Bahlo J, Robrecht S, Langerbeins P, et al. Early treatment with FCR versus watch and wait in patients with stage Binet A high-risk chronic lymphocytic leukemia (CLL): a randomized phase 3 trial. Leukemia. 2020;34(8):2038–50. https://doi.org/10.1038/s41375-020-0747-7.
•• Langerbeins P, Zhang C, Robrecht S, Cramer P, Fürstenau M, Al-Sawaf O, von Tresckow J, et al. The CLL12 trial: ibrutinib vs placebo in treatment-naïve, early-stage chronic lymphocytic leukemia. Blood. 2022;139(2):177–87. https://doi.org/10.1182/blood.2021010845.
Robak P, Robak T. Novel synthetic drugs currently in clinical development for chronic lymphocytic leukemia. Expert Opin Investig Drugs. 2017;26(11):1249–65. https://doi.org/10.1080/13543784.2017.1384814.
Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum K, et al. Ibrutinib treatment for first-line and relapsed/refractory chronic lymphocytic leukemia: final analysis of the pivotal phase Ib/II PCYC-1102 study. Clin Cancer Res. 2020;26(15):3918–27. https://doi.org/10.1158/1078-0432.CCR-19-2856.
Byrd JC, Hillmen P, O'Brien S, Barrientos JC, Reddy NM, Coutre S, et al. Long-term follow-up of the RESONATE phase 3 trial of ibrutinib vs ofatumumab. Blood. 2019;133(19):2031–42. https://doi.org/10.1182/blood-2018-08-870238.
Munir T, Brown JR, O'Brien S, Barrientos JC, Barr PM, Reddy NM, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353–63. https://doi.org/10.1002/ajh.25638.
•• Burger JA, Barr PM, Robak T, Owen C, Ghia P, Tedeschi A, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787–98. https://doi.org/10.1038/s41375-019-0602-x This reference is of outstanding importance because presents important results with long-term follow-up in patients with TN-CLL/SLL treated with ibrutinib.
Hillmen P, Fraser G, Jones J, Rule S, Brien S, Dilhuydy MS, et al. Comparing single-agent ibrutinib, bendamustine plus rituximab (BR) and ibrutinib plus BR in patients with previously-treated chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): an in-direct comparison of the RESONATE and HELIOS trials. Blood. 2015;129:2944. https://doi.org/10.1182/blood.V126.23.2944.2944.
Fraser G, Cramer P, Demirkan F, Silva RS, Grosicki S, Pristupa A, et al. Updated results from the phase 3 HELIOS study of ibrutinib, bendamustine, and rituximab in relapsed chronic lymphocytic leukemia/small lymphocytic lymphoma. Leukemia. 2019;33(4):969–80. https://doi.org/10.1038/s41375-018-0276-9.
Moreno C, Greil R, Demirkan F, Tedeschi A, Anz B, Larratt L, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(1):43–56. https://doi.org/10.1016/S1470-2045(18)30788-5.
•• Shanafelt TD, Wang XV, Kay NE, Hanson CA, O'Brien S, Barrientos J, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432–43. https://doi.org/10.1056/NEJMoa1817073 This reference is of outstanding importance because reveals superior safety and efficacy of ibrutinib-rituximab approach compared with chemoimmunotherapy in CLL patients with unmutated IGHV.
Sharman, J.P.; Egyed,M.; Jurczak,W.; Skarbnik, A.; Pagel, J.M.; Kamdar, M.K.;Munir, T.; Corbett, G.; Fogliatto, L.M.; Herishanu, Y.; et al. Acalabrutinib _ obinutuzumab versus obinutuzumab + chlorambucil in treatment-naïve chronic lymphocytic leukemia: Elevate-TN four-year follow up. J Clin Oncol 2021, 39, 7509.
•• Ghia P, Pluta A, Wach M, Lysak D, Kozak T, Simkovic M, Kaplan, et al. ASCEND: Phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2849–61. https://doi.org/10.1200/JCO.19.03355 This reference is of importance because reveals superior safety and similar efficacy of acalabrutinib compared with ibrutinib in RR-CLL/SLL.
Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocyticleukemia. N Engl J Med. 2016;374(4):311–22. https://doi.org/10.1056/NEJMoa1513257.
Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D'Rozario J, Assouline S, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107–20. https://doi.org/10.1056/NEJMoa1713976.
Kater AP, Wu JQ, Kipps T, Eichhorst B, Hillmen P, D'Rozario J, et al. Venetoclax plus rituximab in relapsed chronic lymphocytic leukemia: 4-year results and evaluation of impact of genomic complexity and gene mutations from the MURANO phase III study. J Clin Oncol. 2020;38(34):4042–54. https://doi.org/10.1200/JCO.20.00948.
•• Fischer K, Al-Sawaf O, Bahlo J, Fink A-M, Tandon M, Dixon M, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225–36. https://doi.org/10.1056/NEJMoa1815281 This reference is of importance because reveals good safety and efficacy of therapy with venetoclax and obinutuzumab in unfit patients with CLL/SLL.
Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. https://doi.org/10.1056/nejmoa1315226.
Flinn IW, Hillmen P, Montillo M, Nagy Z, Illés Á, Etienne G, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132:2446–55. https://doi.org/10.1182/blood-2018-05-850461.
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–37. https://doi.org/10.1056/NEJMoa1509388.
Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner-Johnston ND, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123:3390–7. https://doi.org/10.1182/blood-2013-11-535047.
Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323–32. https://doi.org/10.1056/NEJMoa1509981.
Tambaro FP, De Novellis D, Wierda WG. The role of BTK inhibition in the treatment of chronic lymphocytic leukemia: a clinical view. J Exp Pharmacol. 2021;13:923–35. https://doi.org/10.2147/JEP.S265284.
de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ, Pals ST, Spaargaren M. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119(11):2590–4. https://doi.org/10.1182/blood-2011-11-390989.
Robak T, Robak P. BCR signaling in chronic lymphocytic leukemia and related inhibitors currently in clinical studies. Int Rev Immunol. 2013;32(4):358–76. https://doi.org/10.3109/08830185.2013.786711.
Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–28. https://doi.org/10.1056/NEJMoa1812836.
Sharman JP, Brander DM, Mato AR, Ghosh N, Schuster SJ, Kambhampati S, et al. Ublituximab plus ibrutinib versus ibrutinib alone for patients with relapsed or refractory high-risk chronic lymphocytic leukaemia (GENUINE): a phase 3, multicentre, open-label, randomised trial. Lancet Haematol. 2021;8:e254–e66. https://doi.org/10.1016/S2352-3026(20)30433-6.
•• Byrd JC, Hillmen P, Ghia P, Kater AP, Chanan-Khan A, Furman RR, et al. acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;39(31):3441–52. https://doi.org/10.1200/JCO.21.01210 This reference is of importance because reveals superior safety and similar efficacy of acalabrutinib compared with ibrutinib in RR-CLL/SLL.
•• Hillmen P, Brown JR, Eichhorst BF, Lamanna N, O'Brien SM, Qiu L, et al. ALPINE: zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Future Oncol. 2020;16(10):517–23. https://doi.org/10.2217/fon-2019-0844 This reference is of importance because reveals superior safety and efficacy of zanubrutinib compared with ibrutinib in RR-CLL/SLL.
Hillmen P, Eichhorst B, Brown JR, Lamanna N, O'Brien S, Tam CS, et al. First interim analysis of alpine study: results of a phase 3 randomized study of zanubrutinib vs ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. EHA Library. 06/11/21; 330170; LB1900.
Tam C, Giannopoulos K, Jurczak W, Šimkovič M, Shadman M, Österborg A, et al. SEQUOIA: results of a phase 3 randomized study of zanubrutinib versus bendamustine + rituximab (BR) in patients with treatment-naïve (TN) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Blood 2021;138: (Suppl. 1), Abstract 396). https://doi.org/10.1182/blood-2021-148457
Allan JN, Shanafelt T, Wiestner A, Moreno C, O'Brien SM, Li J, et al. Long-term efficacy of first-line ibrutinib treatment for chronic lymphocytic leukaemia in patients with TP53 aberrations: a pooled analysis from four clinical trials. Br J Haematol. 2022;196(4):947–53. https://doi.org/10.1111/bjh.17984.
•• Jain N, Keating M, Thompson P, Ferrajoli A, Burger J, Borthakur G, et al. Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med. 2019;380(22):2095–103. https://doi.org/10.1056/NEJMoa1900574 This reference is of importance because presented promissing results of combinig therapy with BTK- and BCL2 inhibitors as a first-line therapy.
Kater A, Owen C, Moreno C, Follows G, Munir T., Levin MD, et al. Fixed-dyration ibrutinib and venetoclax (I+V) versus chlorambucil plus Obinutuzumab (CLB+O) for first line chronic lymphocytoc leukemia: primary analysis of the phase 3 GLOW study. EHA 2021. Abstract: LB1902.
Fürstenau M, Eichhorst B. Novel agents in chronic lymphocytic leukemia: new combination therapies and strategies to overcome resistance. Cancers (Basel). 2021;13(6):1336. https://doi.org/10.3390/cancers13061336.
Roberts AW, Ma S, Kipps TJ, Coutre SE, Davids MS, Eichhorst B, et al. Efficacy of venetoclax in relapsed chronic lymphocytic leukemia is influenced by disease and response variables. Blood. 2019;134(2):111–22. https://doi.org/10.1182/blood.2018882555.
O'Brien S, Furman RR, Coutre S, Flinn IW, Burger JA, Blum K, et al. Single-agent ibrutinib in treatment-naïve and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;131(17):1910–9. https://doi.org/10.1182/blood-2017-10-810044.
Fischer K, Bahlo J, Fink AM, Goede V, Herling CD, Cramer P, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208–15. https://doi.org/10.1182/blood-2015-06-651125.
Burger JA, Sivina M, Jain N, Kim E, Kadia T, Estrov Z, et al. Randomized trial of ibrutinib vs ibrutinib plus rituximab in patients with chronic lymphocytic leukemia. Blood. 2019;133(10):1011–9. https://doi.org/10.1182/blood-2018-10-879429.
Collett L, Howard DR, Munir T, McParland L, Oughton JB, Rawstron AC, et al. Assessment of ibrutinib plus rituximab in front-line CLL (FLAIR trial): study protocol for a phase III randomised controlled trial. Trials. 2017;18(1):387. https://doi.org/10.1186/s13063-017-2138-6.
Pitchford A , Bloor A, Broom A, Young M, Kennedy B, Walewska R et al.. Ibrutinib plus rituximab is superior to FCR in previously untreated CLL: results of the Phase III NCRI FLAIR Trial. Blood 2021; 138 (Suppl): Abstract 642.
Davids MS, Brander DM, Kim HT, Tyekucheva S, Bsat J, Savell A, et al.; Blood Cancer Research Partnership of the Leukemia & Lymphoma Society. Ibrutinib plus fludarabine, cyclophosphamide, and rituximab as initial treatment for younger patients with chronic lymphocytic leukaemia: a single-arm, multicentre, phase 2 trial. Lancet Haematol. 2019 A;6(8):e419-e28. https://doi.org/10.1016/S2352-3026(19)30104-8.
Jain N, Thompson P, Burger J, Ferrajoli A, Takahashi K, Estrov Z, et al. Ibrutinib, fludarabine, cyclophosphamide, and obinutuzumab (iFCG) regimen for chronic lymphocytic leukemia (CLL) with mutated IGHV and without TP53 aberrations. Leukemia. 2021;35(12):3421–9. https://doi.org/10.1038/s41375-021-01280-8.
Davids MS, Brander DM, Kim HT, Tyekucheva S, Bsat J, Savell A, et al. Ibrutinib plus fludarabine, cyclophosphamide, and rituximab as initial treatment for younger patients with chronic lymphocytic leukaemia: a single-arm, multicentre, phase 2 trial. Lancet Haematol. 2019;6(8):e419–28. https://doi.org/10.1016/S2352-3026(19)30104-8.
Herman SEM, Montraveta A, Niemann CU, Mora-Jensen H, Gulrajani M, Krantz F, Mantel R, Smith LL, McClanahan F, Harrington BK, Colomer D, Covey T, Byrd JC, Izumi R, Kaptein A, Ulrich R, Johnson AJ, Lannutti BJ, Wiestner A, Woyach JA. The Bruton tyrosine kinase (BTK) inhibitor acalabrutinib demonstrates potent on-target effects and efficacy in two mouse models of chronic lymphocytic leukemia. Clin Cancer Res. 2017;23(11):2831–41. https://doi.org/10.1158/1078-0432.CCR-16-0463.
Li N, Sun Z, Liu Y, Guo M, Zhang Y, Zhou D. BGB-3111 is a novel and highly selective Bruton’s tyrosine kinase (BTK) inhibitor [Abstract]. Cancer Res. 2015;75:2597. https://doi.org/10.1158/1538-7445.AM2015-2597.
Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851–9. https://doi.org/10.1182/blood.2019001160.
Xu W, Yang S, Zhou K, Pan L, Li Z, Zhou J, et al. Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single-arm, multicenter study. J Hematol Oncol. 2020;13(1):48. https://doi.org/10.1186/s13045-020-00884-4.
•• Tam CS, Robak T, Ghia P, Kahl BS, Walker P, Janowski W, et al. Zanubrutinib monotherapy for patients with treatment naïve chronic lymphocytic leukemia and 17p deletion. Haematologica. 2020;106(9):2354–63. https://doi.org/10.3324/haematol.2020.259432 This reference is of importance because presents promising results of zanabrutinib monotherapy in TN-CLL/SLL with deletion17p.
•• Robak T, Witkowska M, Smolewski P. The role of Bruton’s kinase inhibitors in chronic lymphocytic leukemia: current status and future directions. Cancers (Basel). 2022;14:771. https://doi.org/10.3390/cancers14030771 This reference is of importance because presents current knowledge about BTK inhibitors.
Gomez EB, Isabel L, Rosendahal MS, Rothenberg SM, Andrews SW, Brandhuber BJ. Loxo-305, a highly selective and non-covalent next generation BTK inhibitor, inhibits diverse BTK C481 substitution mutations. Blood. 2019;134(Supplement_1):4644. https://doi.org/10.1182/blood-2019-126114.
•• Mato AR, Shah NN, Jurczak W, Cheah CY, Pagel JM, Woyach JA, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021;397(10277):892–901. https://doi.org/10.1016/S0140-6736(21)00224-5 This reference is of importance because reveals promising results of next-generation BTK inhibitor—pirtobrutinib in RR-CLL/SLL.
Jurczak W, Shah NN, Lamanna N, Eyre TA, Woyach J, Lech-Maranda E, et al. Pirtobrutinib (loxo-305), a next generation highly selective non-covalent btk inhibitor in previously treated Richter transformation: results from the phase 1/2 bruin study. Hematol Oncol. 2021;39(S2). https://doi.org/10.1002/hon.41_2880.
Woyach J, Stephens DM, Flinn IW, Bhat SA, Savage RE, Chai F, et al. Final results of phase 1, dose escalation study evaluating ARQ 531 in patients with relapsed or refractory B-cell lymphoid malignancies. Blood. 2019;134(Supplement 1):4298. https://doi.org/10.1182/blood-2019-127260.
Sharman JP, Coutre SE, Furman RR, Cheson BD, Pagel JM, Hillmen P, et al. Final results of a randomized, phase III study of rituximab with or without idelalisib followed by open-label idelalisib in patients with relapsed chronic lymphocytic leukemia. J Clin Oncol. 2019;37:1391–402. https://doi.org/10.1200/JCO.18.01460.
Jones JA, Robak T, Brown JR, Awan FT, Badoux X, Coutre S, et al. Efficacy and safety of idelalisib in combination with ofatumumab for previously treated chronic lymphocytic leukaemia: an open-label, randomised phase 3 trial. Lancet Haematol. 2017;4(3):e114–e26. https://doi.org/10.1016/S2352-3026(17)30019-4.
Zelenetz AD, Barrientos JC, Brown JR, Coiffier B, Delgado J, Egyed M, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2017;18:297–311. https://doi.org/10.1016/S1470-2045(16)30671-4.
Lampson BL, Kasar SN, Matos TR, Morgan EA, Rassenti L, Davids MS, et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood. 2016;128(2):195–203. https://doi.org/10.1182/blood-2016-03-707133.
Tarantelli C, Argnani L, Zinzani PL, Bertoni F. PI3Kδ inhibitors as immunomodulatory agents for the treatment of lymphoma patients. Cancers (Basel). 2021;13:5535. https://doi.org/10.3390/cancers13215535.
Vogler M, Dinsdale D, Dyer MJ, Cohen GM. ABT-199 selectively inhibits BCL2 but not BCL2L1 and efficiently induces apoptosis of chronic lymphocytic leukaemic cells but not platelets. Br J Haematol. 2013;163(1):139–42. https://doi.org/10.1111/bjh.12457.
Stilgenbauer S, Eichhorst B, Schetelig J, Hillmen P, Seymour JF, Coutre S, et al. Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: results from the full population of a phase II pivotal trial. J Clin Oncol. 2018;36(19):1973–80. https://doi.org/10.1200/JCO.2017.76.6840.
Korycka-Wolowiec A, Wolowiec D, Kubiak-Mlonka A, Robak T. Venetoclax in the treatment of chronic lymphocytic leukemia. Expert Opin Drug Metab Toxicol. 2019;15(5):353–66. https://doi.org/10.1080/17425255.2019.1606211.61.
Iskierka-Jażdżewska E, Robak T. Investigational treatments for chronic lymphocytic leukemia: a focus on phase 1 and 2 clinical trials. Expert Opin Investig Drugs. 2020;29(7):709–22. https://doi.org/10.1080/13543784.2020.1770225.
Al-Sawaf O, Zhang C, Tandon M, Sinha A, Fink AM, Robrecht S, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21(9):1188–200. https://doi.org/10.1016/S1470-2045(20)30443-5.
Munir T, Moreno C, Owen C, Follows GA, Benjamini O, Janssens A, et al. First prospective data on minimal residual disease (MRD) outcomes after fixed-duration ibrutinib plus venetoclax (Ibr+Ven) versus chlorambucil plus obinutuzumab (Clb+O) for first-line treatment of CLL in elderly or unfit patients: the Glow Study. Blood 2021; 138 (Suppl): Abstract 70.
Cervantes-Gomez F, Lamothe B, Woyach JA, Wierda WG, Keating MJ, Balakrishnan K, et al. Pharmacological and protein profiling suggests venetoclax (ABT-199) as optimal partner with ibrutinib in chronic lymphocytic leukemia. Clin Cancer Res. 2015;21:3705–15.11. https://doi.org/10.1158/1078-0432.CCR-14-2809.
Deng J, Isik E, Fernandes SM, Brown JR, Letai A, Davids MS. Bruton’s tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lymphocytic leukemia. Leukemia. 2017;31:2075–84. https://doi.org/10.1038/leu.2017.32.
Hillmen P, Brown JR, Byrd JC, Eichhorst B, Lamanna N, O’Brien SM, et al. ALPINE: phase III zanubrutinib (BGB-3111) versus ibrutinib in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). J Clin Oncol. 2019;37(Suppl. 15):TPS7572. https://doi.org/10.1200/JCO.2019.37.15_suppl.TPS7572.
Jain N, Keating M, Thompson P, Ferrajoli A, Burger J, Borthakur G, et al. Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med. 2019;380(22):2095–103. https://doi.org/10.1056/NEJMoa1900574.
Wierda WG, Tam CS, Allan JN, Siddiqi T, Kipps TJ, Opat S, et al. Ibrutinib (Ibr) plus venetoclax (Ven) for first-line treatment of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL): 1-year disease-free survival (DFS): results from the MRD cohort of the phase 2 CAPTIVATE Study. Blood. 2020;136:16–7. https://doi.org/10.1182/blood-2020-134446.
Rogers KA, Huang Y, Ruppert AS, Abruzzo LV, Andersen BL, Awan FT, et al. Phase II study of combination obinutuzumab, ibrutinib, and venetoclax in treatment-naïve and relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38:3626–37. https://doi.org/10.1200/JCO.20.00491.
Huber H, Edenhofer S, von Tresckow J, Robrecht S, Zhang C, Tausch E, et al. Phase 2 study of obinutuzumab (GA-101), ibrutinib and venetoclax (CLL2-GIVe) in patients with untreated high-risk chronic lymphocytic leukemia. Blood. 2021 Dec 15:blood.2021013208. https://doi.org/10.1182/blood.2021013208
•• Davids MS, Lampson BL, Tyekucheva S, Wang Z, Pazienza S, Montegaard J, et al. Acalabrutinib, venetoclax, and obinutuzumab as frontline treatment for chronic lymphocytic leukaemia: a single-arm, open-label, phase 2 study. Lancet Oncol. 2021;22:1391–402. https://doi.org/10.1016/S1470-2045(21)00455-1 This reference is of importance because presents important results of combining therapy with acalabrutinib, venetoclax, and obinutuzumab in TN-CLL.
Tedeschi A, Ferrant E, Flinn IW, Tam CS, Ghia P, Robak T, et al. Zanubrutinib in combination with venetoclax for patients with treatment-naïve (TN) chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) with del(17p): early results from Arm D of the SEQUOIA (BGB-3111-304) trial. Blood 2021; 138 (Suppl): Abstract 67.
Soumerai JD, Mato AR, Dogan A, Seshan VE, Joffe E, Flaherty K, et al. Zanubrutinib, obinutuzumab, and venetoclax with minimal residual disease-driven discontinuation in previously untreated patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2021;8(12):e879–e90. https://doi.org/10.1016/S2352-3026(21)00307-0.
Acknowledgments
We thank Edward Lowczowski from the Medical University of Lodz, Poland for editorial assistance.
Funding
This research received no external funding
Author information
Authors and Affiliations
Contributions
All authors contributed to the manuscript conception. Dr Iskierka-Jażdżewska and Prof. Robak had the idea for the manuscript; Dr Iskierka-Jażdżewska , Dr Urbaniak, and Dr. Obracaj performed the literature search and drafted the manuscript, and Prof. Robak critically revised the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr Iskierka-Jażdżewska declares that she served as a consultant for Abbvie, AstaZeneca, Sandoz, Novartis and received honoraria and research funding from Roche, Abbvie, AstaZeneca, Sandoz, Takeda, and Regeneron, outside the submitted work, Dr Obracaj and Dr Urbaniak declares that there is no conflict of interest. Prof. Robak declares that he served as a consultant for Janssen, Abbvie, BeiGene, AstaZeneca, Giliad, and Octapharma and received honoraria and research funding from Janssen, Abbvie, BeiGene, AstaZeneca, Octapharma, GSK, Karyopharm, Sanofi, Takeda, and Regeneron, outside the submitted work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Leukemia
Rights and permissions
About this article
Cite this article
Iskierka-Jażdżewska, E., Obracaj, A., Urbaniak, M. et al. New Treatment Options for Newly-Diagnosed and Relapsed Chronic Lymphocytic Leukemia. Curr. Treat. Options in Oncol. 23, 775–795 (2022). https://doi.org/10.1007/s11864-022-00974-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-022-00974-0