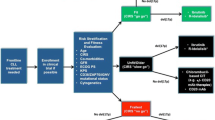
Opinion statement
A number of new treatment options have recently emerged for chronic lymphocytic leukemia (CLL) patients, including the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib, phosphatidylinositol-3-kinase (PI3K) delta isoform inhibitor idelalisib combined with rituximab, the Bcl-2 antagonist venetoclax, and the new anti-CD20 antibodies obinutuzumab and ofatumumab. Most of these agents are already included into treatment algorithms defined by international practice guidelines, but more clinical investigations are needed to answer still remaining questions. Ibrutinib was proven as a primary choice for patients with the TP53 gene deletion/mutation, who otherwise have no active treatment available. Idelalisib with rituximab is also an active therapy, but due to increased risk of serious infections, its use in first-line treatment is limited to patients for whom ibrutinib is not an option. A new indication for ibrutinib was recently approved for older patients with comorbidities, as an alternative to the already existing indication for chlorambucil with obinutuzumab. The use of kinase inhibitors is already well established in recurrent/refractory disease. Immunochemotherapy with fludarabine, cyclophosphamide, rituximab (FCR) remains a major first-line option for many CLL patients without the TP53 gene deletion/mutation, and who have no significant comorbidities or history of infections, and is particularly effective in patients with favorable features including mutated IGHV status. There are a number of issues regarding novel therapies for CLL that need further investigation such as optimum duration of treatment with kinase inhibitors, appropriate sequencing of novel agents, mechanisms of resistance to inhibitors and response to class switching after treatment failure, along with the potential role of combinations of targeted agents.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Rawstron AC, Bennett FL, O’Connor SJ, Kwok M, Fenton JA, Plummer M, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359(6):575–83.
Redaelli A, Laskin BL, Stephens JM, Botteman MF, Pashos CL. The clinical and epidemiological burden of chronic lymphocytic leukaemia. European journal of cancer care. 2004;13(3):279–87.
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–56.
Jamroziak K, Tadmor T, Robak T, Polliack A. Richter syndrome in chronic lymphocytic leukemia: updates on biology, clinical features and therapy. Leukemia & lymphoma. 2015;56(7):1949–58.
Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet (London, England). 2010;376(9747):1164–74.
•• Fischer K, Bahlo J, Fink AM, Goede V, Herling CD, Cramer P, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208–15. Update on the randomized study after a median nearly 6 year follow up confirming sustained PFS and OS advantage to FCR arm mostly seen in IGHV mutated disease
•• Eichhorst B, Fink AM, Bahlo J, Busch R, Kovacs G, Maurer C, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. The Lancet Oncology. 2016;17(7):928–42. Randomized study confirming FCR as a standard initial therapy for fit patients
Wierda WG, Kipps TJ, Mayer J, Stilgenbauer S, Williams CD, Hellmann A, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(10):1749–55.
•• Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–10. Practice changing randomized study in older patients with significant comorbidities demonstrating improvement in OS and PFS with obinutuzumab versus rituximab both compined with chlorambucil
•• Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–23. Practice changing randomized study demonstrating high activity of ibrutinib in recurrent disease compared to ofatumumab
Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. Practice changing placebo-controlled randomized study proving high activity of idelalisb in recurrent disease
•• Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311–22. Phase 1 dose escalation study showing substantial activity of BCL2 inhibitor in relapsed/refractory disease including patients with poor prognostic features
Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46(2):219–34.
Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48(1):198–206.
Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840–7.
Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94(6):1848–54.
Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910–6.
Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, Rozman M, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348(18):1764–75.
Weinberg JB, Volkheimer AD, Chen Y, Beasley BE, Jiang N, Lanasa MC, et al. Clinical and molecular predictors of disease severity and survival in chronic lymphocytic leukemia. Am J Hematol. 2007;82(12):1063–70.
Gattei V, Bulian P, Del Principe MI, Zucchetto A, Maurillo L, Buccisano F, et al. Relevance of CD49d protein expression as overall survival and progressive disease prognosticator in chronic lymphocytic leukemia. Blood. 2008;111(2):865–73.
Agathangelidis A, Darzentas N, Hadzidimitriou A, Brochet X, Murray F, Yan XJ, et al. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: a molecular classification with implications for targeted therapies. Blood. 2012;119(19):4467–75.
Wang L, Lawrence MS, Wan Y, Stojanov P, Sougnez C, Stevenson K, et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N Engl J Med. 2011;365(26):2497–506.
Quesada V, Conde L, Villamor N, Ordonez GR, Jares P, Bassaganyas L, et al. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat Genet. 2011;44(1):47–52.
Puente XS, Pinyol M, Quesada V, Conde L, Ordonez GR, Villamor N, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475(7354):101–5.
Rossi D, Rasi S, Fabbri G, Spina V, Fangazio M, Forconi F, et al. Mutations of NOTCH1 are an independent predictor of survival in chronic lymphocytic leukemia. Blood. 2012;119(2):521–9.
Balatti V, Bottoni A, Palamarchuk A, Alder H, Rassenti LZ, Kipps TJ, et al. NOTCH1 mutations in CLL associated with trisomy 12. Blood. 2012;119(2):329–31.
Landau DA, Carter SL, Stojanov P, McKenna A, Stevenson K, Lawrence MS, et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152(4):714–26.
Landau DA, Tausch E, Taylor-Weiner AN, Stewart C, Reiter JG, Bahlo J, et al. Mutations driving CLL and their evolution in progression and relapse. Nature. 2015;526(7574):525–30.
Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the cumulative illness rating scale. Psychiatry Res. 1992;41(3):237–48.
Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51.
•• Pflug N, Bahlo J, Shanafelt TD, Eichhorst BF, Bergmann MA, Elter T, et al. Development of a comprehensive prognostic index for patients with chronic lymphocytic leukemia. Blood. 2014;124(1):49–62. The authors propose and validate a comprehensive CLL prognostic index with high discriminatory power and prognostic significance based on data from phase 3 trials of the German CLL Study Group
da Cunha-Bang C, Christiansen, I., Niemann, C. U.. The International prognostic Index for patients with chronic lymphocytic leukemia (CLL-IPI) applied in a population-based cohort. Blood. 2016.
Rawstron AC, Fazi C, Agathangelidis A, Villamor N, Letestu R, Nomdedeu J, et al. A complementary role of multiparameter flow cytometry and high-throughput sequencing for minimal residual disease detection in chronic lymphocytic leukemia: an European research initiative on CLL study. Leukemia. 2016;30(4):929–36.
Kovacs, G., Robrecht, S., Fink, A. M., Bahlo, J., Cramer, P., von Tresckow, J., et al. Minimal residual disease assessment improves prediction of outcome in patients with chronic lymphocytic leukemia (CLL) who achieve partial response: comprehensive analysis of two phase III studies of the German CLL study group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016.
Teeling JL, French RR, Cragg MS, van den Brakel J, Pluyter M, Huang H, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104(6):1793–800.
Teeling JL, Mackus WJ, Wiegman LJ, van den Brakel JH, Beers SA, French RR, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. Journal of immunology (Baltimore, Md : 1950). 2006;177(1):362–71.
Pawluczkowycz AW, Beurskens FJ, Beum PV, Lindorfer MA, van de Winkel JG, Parren PW, et al. Binding of submaximal C1q promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): considerably higher levels of CDC are induced by OFA than by RTX. Journal of immunology (Baltimore, Md : 1950). 2009;183(1):749–58.
Coiffier B, Losic N, Ronn BB, Lepretre S, Pedersen LM, Gadeberg O, et al. Pharmacokinetics and pharmacokinetic/pharmacodynamic associations of ofatumumab, a human monoclonal CD20 antibody, in patients with relapsed or refractory chronic lymphocytic leukaemia: a phase 1-2 study. Br J Haematol. 2010;150(1):58–71.
Coiffier B, Lepretre S, Pedersen LM, Gadeberg O, Fredriksen H, van Oers MH, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1-2 study. Blood. 2008;111(3):1094–100.
Moreno C, Montillo M, Panayiotidis P, Dimou M, Bloor A, Dupuis J, et al. Ofatumumab in poor-prognosis chronic lymphocytic leukemia: a phase IV, non-interventional, observational study from the European research initiative on chronic lymphocytic leukemia. Haematologica. 2015;100(4):511–6.
Doubek M, Brychtova Y, Panovska A, Sebejova L, Stehlikova O, Chovancova J, et al. Ofatumumab added to dexamethasone in patients with relapsed or refractory chronic lymphocytic leukemia: results from a phase II study. Am J Hematol. 2015;90(5):417–21.
Cortelezzi A, Sciume M, Liberati AM, Vincenti D, Cuneo A, Reda G, et al. Bendamustine in combination with ofatumumab in relapsed or refractory chronic lymphocytic leukemia: a GIMEMA multicenter phase II trial. Leukemia. 2014;28(3):642–8.
Ujjani C, Ramzi P, Gehan E, Wang H, Wang Y, Cheson BD. Ofatumumab and bendamustine in previously treated chronic lymphocytic leukemia and small lymphocytic lymphoma. Leukemia & lymphoma. 2015;56(4):915–20.
Flinn IW, Panayiotidis P, Afanasyev B, Janssens A, Grosicki S, Homenda W, et al. A phase 2, multicenter study investigating ofatumumab and bendamustine combination in patients with untreated or relapsed CLL. Am J Hematol. 2016;91(9):900–6.
Hillmen P, Robak T, Janssens A, Babu KG, Kloczko J, Grosicki S, et al. Chlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukaemia (COMPLEMENT 1): a randomised, multicentre, open-label phase 3 trial. Lancet (London, England). 2015;385(9980):1873–83.
Hillmen P, Janssens A, Babu KG, Kloczko J, Grosicki S, Manson S, et al. Health-related quality of life and patient-reported outcomes of ofatumumab plus chlorambucil versus chlorambucil monotherapy in the COMPLEMENT 1 trial of patients with previously untreated CLL. Acta oncologica (Stockholm, Sweden). 2016;55(9–10):1115–20.
Tedeschi A, Rossi D, Motta M, Quaresmini G, Rossi M, Coscia M, et al. A phase II multi-center trial of pentostatin plus cyclophosphamide with ofatumumab in older previously untreated chronic lymphocytic leukemia patients. Haematologica. 2015;100(12):e501–4.
Strati P, Lanasa M, Call TG, Leis JF, Brander DM, LaPlant BR, et al. Ofatumumab monotherapy as a consolidation strategy in patients with previously untreated chronic lymphocytic leukaemia: a phase 2 trial. The Lancet Haematology. 2016;3(9):e407–14.
Ahn I, Farooqui M, Lee YS, Marti G, Soto S, Tian X, et al. Risk-adapted induction and maintenance with ofatumumab in previously untreated patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). Blood. 2015;126(23):1750.
van Oers MH, Kuliczkowski K, Smolej L, Petrini M, Offner F, Grosicki S, et al. Ofatumumab maintenance versus observation in relapsed chronic lymphocytic leukaemia (PROLONG): an open-label, multicentre, randomised phase 3 study. The Lancet Oncology. 2015;16(13):1370–9.
Wierda WG, Kipps TJ, Durig J, Griskevicius L, Stilgenbauer S, Mayer J, et al. Chemoimmunotherapy with O-FC in previously untreated patients with chronic lymphocytic leukemia. Blood. 2011;117(24):6450–8.
Flinn IW, Ruppert AS, Harwin W, Waterhouse D, Papish S, Jones JA, et al. A phase II study of two dose levels of ofatumumab induction followed by maintenance therapy in symptomatic, previously untreated chronic lymphocytic leukemia. Am J Hematol. 2016;91(10):1020–5.
Costa LJ, Fanning SR, Stephenson Jr J, Afrin LB, Kistner-Griffin E, Bentz TA, et al. Sequential ofatumumab and lenalidomide for the treatment of relapsed and refractory chronic lymphocytic leukemia and small lymphocytic lymphoma. Leukemia & lymphoma. 2015;56(3):645–9.
Vitale C, Falchi L, Ten Hacken E, Gao H, Shaim H, Van Roosbroeck K, et al. Ofatumumab and lenalidomide for patients with relapsed or refractory chronic lymphocytic leukemia: correlation between responses and immune characteristics. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(10):2359–67.
Golay J, Da Roit F, Bologna L, Ferrara C, Leusen JH, Rambaldi A, et al. Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood. 2013;122(20):3482–91.
Niederfellner G, Lammens A, Mundigl O, Georges GJ, Schaefer W, Schwaiger M, et al. Epitope characterization and crystal structure of GA101 provide insights into the molecular basis for type I/II distinction of CD20 antibodies. Blood. 2011;118(2):358–67.
Mossner E, Brunker P, Moser S, Puntener U, Schmidt C, Herter S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115(22):4393–402.
Goede V, Fischer K, Engelke A, Schlag R, Lepretre S, Montero LF, et al. Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: updated results of the CLL11 study. Leukemia. 2015;29(7):1602–4.
Byrd JC, Flynn JM, Kipps TJ, Boxer M, Kolibaba KS, Carlile DJ, et al. Randomized phase 2 study of obinutuzumab monotherapy in symptomatic, previously untreated chronic lymphocytic leukemia. Blood. 2016;127(1):79–86.
Brown JR, O’Brien S, Kingsley CD, Eradat H, Pagel JM, Lymp J, et al. Obinutuzumab plus fludarabine/cyclophosphamide or bendamustine in the initial therapy of CLL patients: the phase 1b GALTON trial. Blood. 2015;125(18):2779–85.
Le Garff-Tavernier M, Herbi L, de Romeuf C, Nguyen-Khac F, Davi F, Grelier A, et al. Antibody-dependent cellular cytotoxicity of the optimized anti-CD20 monoclonal antibody ublituximab on chronic lymphocytic leukemia cells with the 17p deletion. Leukemia. 2014;28(1):230–3.
Ben Abdelwahed R, Donnou S, Ouakrim H, Crozet L, Cosette J, Jacquet A, et al. Preclinical study of ublituximab, a Glycoengineered anti-human CD20 antibody, in murine models of primary cerebral and intraocular B-cell lymphomas. Invest Ophthalmol Vis Sci. 2013;54(5):3657–65.
O’Connor, O. A., Schreeder, M. T., Deng, C., Amengual, J. E., Boccia, R., Khalil, M. Y., et al. Ublituximab (TG-1101), a novel anti-CD20 monoclonal antibody for rituximab relapsed/refractory B-cell malignancies. European Hematology Association (EHA); Milan, Italy 2014.
Lunning MA, Vose J, Fowler N, Nastoupil L, Burger JA, Wierda W, et al. Ublituximab + TGR-1202 demonstrates activity and a favorable safety profile in relapsed/refractory B-cell NHL and high-risk CLL: phase I results. Blood. 2015;126(23):1538.
Woyach JA, Bojnik E, Ruppert AS, Stefanovski MR, Goettl VM, Smucker KA, et al. Bruton’s tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL). Blood. 2014;123(8):1207–13.
Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117(23):6287–96.
Herman SE, Mustafa RZ, Gyamfi JA, Pittaluga S, Chang S, Chang B, et al. Ibrutinib inhibits BCR and NF-kappaB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood. 2014;123(21):3286–95.
Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(1):88–94.
Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42.
Sun C, Tian X, Lee YS, Gunti S, Lipsky A, Herman SE, et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood. 2015;126(19):2213–9.
Farooqui MZ, Valdez J, Martyr S, Aue G, Saba N, Niemann CU, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. The Lancet Oncology. 2015;16(2):169–76.
Wanquet A, Birsen R, Lemal R, Hunault M, Leblond V, Aurran-Schleinitz T. Ibrutinib responsive central nervous system involvement in chronic lymphocytic leukemia. Blood. 2016;127(19):2356–8.
O’Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. The Lancet Oncology. 2014;15(1):48–58.
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–37.
Burger JA, Keating MJ, Wierda WG, Hartmann E, Hoellenriegel J, Rosin NY, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. The Lancet Oncology. 2014;15(10):1090–9.
Jaglowski SM, Jones JA, Nagar V, Flynn JM, Andritsos LA, Maddocks KJ, et al. Safety and activity of BTK inhibitor ibrutinib combined with ofatumumab in chronic lymphocytic leukemia: a phase 1b/2 study. Blood. 2015;126(7):842–50.
Brown JR, Barrientos JC, Barr PM, Flinn IW, Burger JA, Tran A, et al. The Bruton tyrosine kinase inhibitor ibrutinib with chemoimmunotherapy in patients with chronic lymphocytic leukemia. Blood. 2015;125(19):2915–22.
Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. The Lancet Oncology. 2016;17(2):200–11.
Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107(29):13075–80.
Byrd JC, Harrington B, O’Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323–32.
Walter HS, Rule SA, Dyer MJ, Karlin L, Jones C, Cazin B, et al. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood. 2016;127(4):411–9.
Vanhaesebroeck B, Khwaja A. PI3Kdelta inhibition hits a sensitive spot in B cell malignancies. Cancer Cell. 2014;25(3):269–71.
Herman SE, Gordon AL, Wagner AJ, Heerema NA, Zhao W, Flynn JM, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078–88.
Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117(2):591–4.
Davids MS, Deng J, Wiestner A, Lannutti BJ, Wang L, Wu CJ, et al. Decreased mitochondrial apoptotic priming underlies stroma-mediated treatment resistance in chronic lymphocytic leukemia. Blood. 2012;120(17):3501–9.
Fiorcari S, Brown WS, McIntyre BW, Estrov Z, Maffei R, O’Brien S, et al. The PI3-kinase delta inhibitor idelalisib (GS-1101) targets integrin-mediated adhesion of chronic lymphocytic leukemia (CLL) cell to endothelial and marrow stromal cells. PLoS One. 2013;8(12):e83830.
Maffei R, Bulgarelli J, Fiorcari S, Martinelli S, Castelli I, Valenti V, et al. Endothelin-1 promotes survival and chemoresistance in chronic lymphocytic leukemia B cells through ETA receptor. PLoS One. 2014;9(6):e98818.
Ghia, P., Cheson, B. D., Furman, R. R., Hallek, M., Hillmen, P., O’Brien, S. M., et al., (eds). Patterns of lymphocytosis in patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) treated with idelalisib. European Hematology Association Annual Meeting; 2015; Copenhagen, Denmark.
Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner-Johnston ND, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390–7.
Barrientos JC, Coutre S, De Vos S, Flinn I, Wagner-Johnston ND, Schreeder MT, et al. Long-term follow-up of a phase Ib trial of idelalisib (IDELA) in combination with chemoimmunotherapy (CIT) in patients (pts) with relapsed/refractory (R/R) CLL including pts with del17p/TP53 mutation. J Clin Oncol. 2015;33(Suppl) Abstr:7011.
Jones JA, Wach M, Robak T, Brown JR, Menter AR, Vandenberghe E, et al. Results of a phase III randomized, controlled study evaluating the efficacy and safety of idelalisib (IDELA) in combination with ofatumumab (OFA) for previously treated chronic lymphocytic leukemia (CLL). J Clin Oncol. 2015;33:7023.
O’Brien SM, Lamanna N, Kipps TJ, Flinn I, Zelenetz AD, Burger JA, et al. A phase 2 study of idelalisib plus rituximab in treatment-naive older patients with chronic lymphocytic leukemia. Blood. 2015;126(25):2686–94.
Lampson, B. L., Kasar, S. N., Matos, T. R., Morgan, E. A., Rassenti, L., Davids, M. S., et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood. 2016.
Coutre SE, Barrientos JC, Brown JR, de Vos S, Furman RR, Keating MJ, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leukemia & lymphoma. 2015;56(10):2779–86.
Brown JR. The PI3K pathway: clinical inhibition in chronic lymphocytic leukemia. Semin Oncol. 2016;43(2):260–4.
Graf SA, Gopal AK. Idelalisib for the treatment of non-Hodgkin lymphoma. Expert Opin Pharmacother. 2016;17(2):265–74.
De Vos S, Furman RR, Barrientos JC, Wagner-Johnston ND, Flinn I, Sharman JP, et al. Idelalisib, a selective inhibitor of PI3Kδ, in combination with bendamustine, fludarabine or chlorambucil in patients with relapsed or refractory (R/R) chronic lymphocytic leukemia (CLL). Blood. 2013;122(21):2878.
Cheah CY, Fowler NH. Idelalisib in the management of lymphoma. Blood. 2016;128(3):331–6.
Smith SM, Pitcher B, Jung S-H, Bartlett NL, Wagner-Johnston N, Park SI, et al. Unexpected and serious toxicity observed with combined idelalisib, lenalidomide and rituximab in relapsed/refractory B cell lymphomas: alliance A051201 and A051202. Blood. 2014;124(21):3091.
Cheah CY, Nastoupil LJ, Neelapu SS, Forbes SG, Oki Y, Fowler NH. Lenalidomide, idelalisib, and rituximab are unacceptably toxic in patients with relapsed/refractory indolent lymphoma. Blood. 2015;125(21):3357–9.
Barr PM, Saylors GB, Spurgeon SE, Cheson BD, Greenwald DR, O’Brien SM, et al. Phase 2 study of idelalisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood. 2016;127(20):2411–5.
Thompson PA, Stingo F, Keating MJ, Ferrajoli A, Burger JA, Wierda WG, et al. Outcomes of patients with chronic lymphocytic leukemia treated with first-line idelalisib plus rituximab after cessation of treatment for toxicity. Cancer. 2016;122(16):2505–11.
Balakrishnan K, Peluso M, Fu M, Rosin NY, Burger JA, Wierda WG, et al. The phosphoinositide-3-kinase (PI3K)-delta and gamma inhibitor, IPI-145 (Duvelisib), overcomes signals from the PI3K/AKT/S6 pathway and promotes apoptosis in CLL. Leukemia. 2015;29(9):1811–22.
Horwitz SM, Flinn I, Patel MR, Younes A, Foss FM, Oki Y, et al. Preliminary safety and efficacy of IPI-145, a potent inhibitor of phosphoinositide-3-kinase-δ,γ, in patients with relapsed/refractory lymphoma. J Clin Oncol. 2013;31 :8518.abstr
Porcu P, Flinn I, Kahl BS, Horwitz SM, Oki Y, Byrd JC, et al. Clinical activity of duvelisib (IPI-145), a phosphoinositide-3-kinase-δ,γ inhibitor, in patients previously treated with ibrutinib. Blood. 2014;124(21):3335.
O’Brien S, Patel M, Kahl BS, Horwitz SM, Foss FM, Porcu P, et al. Duvelisib (IPI-145), a PI3K-δ,γ inhibitor, is clinically active in patients with relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;124(21):3334.
Flinn IW, Cherry M, Maris M, Matous JV, Berdeja JG, Patel MR. Combination trial of duvelisib (IPI-145) with bendamustine, rituximab, or bendamustine/rituximab in patients with lymphoma or chronic lymphocytic leukemia. Blood. 2015;126(23):3928.
O’Connor OA, Flinn IW, Patel MR, Fenske TS, Deng C, Brander DM, et al. TGR-1202, a novel once daily PI3K-Delta inhibitor, demonstrates clinical activity with a favorable safety profile in patients with CLL and B-cell lymphoma. Blood. 2015;126(23):4154.
Balakrishnan K, Gandhi V. Bcl-2 antagonists: a proof of concept for CLL therapy. Investig New Drugs. 2013;31(5):1384–94.
Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(5):488–96.
Roberts AW, Ma S, Brander DM, Kipps TJ, Barrientos JC, Davids MS, et al. Venetoclax (ABT-199 / GDC-0199) combined with rituximab induces deep responses in patients with relapsed/refractory chronic lymphocytic leukemia. European Hematology Annual Meeting. 2015;S481
Stilgenbauer S, Eichhorst B, Schetelig J, Coutre S, Seymour JF, Munir T, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. The Lancet Oncology. 2016;17(6):768–78.
Porter DL, Hwang W-T, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139.
Maddocks KJ, Pagel J, Byrd JC, Stromatt S, Awan F. Phase 1b study of otlertuzumab (TRU-016), an anti-CD37 ADAPTIRTM protein, in combination with rituximab in patients with chronic lymphocytic leukemia (CLL). Blood. 2014;124(21):4671.
Ding W, Dong H, Call TG, Shanafelt TD, Parikh SA, Leis JF, et al. PD-1 blockade with pembrolizumab (MK-3475) in relapsed/refractory CLL including Richter transformation: an early efficacy report from a phase 2 trial (MC1485). Blood. 2015;126(23):834.
Kwok M, Davies N, Agathanggelou A, Smith E, Oldreive C, Petermann E, et al. ATR inhibition induces synthetic lethality and overcomes chemoresistance in TP53- or ATM-defective chronic lymphocytic leukemia cells. Blood. 2016;127(5):582–95.
Kwok M, Davies N, Agathanggelou A, Smith E, Petermann E, Yates E, et al. Synthetic lethality in chronic lymphocytic leukaemia with DNA damage response defects by targeting the ATR pathway. Lancet (London, England). 2015;385(Suppl 1):S58.
Eichhorst, B., Robak, T., Montserrat, E., Ghia, P., Hillmen, P., Hallek, M., et al. appendix 6: Chronic lymphocytic leukaemia: eUpdate published online September 2016 (http://www.esmo.org/Guidelines/Haematological-Malignancies). Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2016;27(suppl 5):v143-v4.
Eichhorst B, Robak T, Montserrat E, Ghia P, Hillmen P, Hallek M, et al. Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2015;26(Suppl 5):v78–84.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Chronic lymphocytic leukemia/small lymphocytic lymphoma. Version I. 2017 – September 28, 2016.
Brown, J. R., Hallek, M. J., Pagel, J. M. Chemoimmunotherapy versus targeted treatment in chronic lymphocytic leukemia: when, how long, how much, and in which combination? American Society of Clinical Oncology educational book / ASCO American Society of Clinical Oncology Meeting. 2016;35:e387–98.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Krzysztof Jamroziak has received compensation and from Jannsen-Cilag, Gilead, and AbbVie for service as a consultant and on speakers’ bureaus.
Bartosz Puła declares that he has no conflict of interest.
Jan Walewski has received research support through grants from Roche, Mundipharma, Celgene, and GSK/Novartis and has received compensation from Roche, Mundipharma, Celgene, Takeda, Janssen-Cilag, Teva, Boehringer-Ingelheim, Karyopharm, ARIAD, Servier, Gilead, and Sanofi for service as a consultant, lecture honoraria, and reimbursement of travel costs.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Leukemia
Rights and permissions
About this article
Cite this article
Jamroziak, K., Puła, B. & Walewski, J. Current Treatment of Chronic Lymphocytic Leukemia. Curr. Treat. Options in Oncol. 18, 5 (2017). https://doi.org/10.1007/s11864-017-0448-2
Published:
DOI: https://doi.org/10.1007/s11864-017-0448-2