Opinion statement
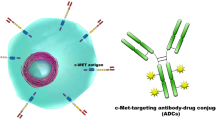
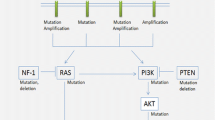
High-grade gliomas remain incurable despite current therapies, which are plagued by high morbidity and mortality. Molecular categorization of glioma subtypes using mutations in isocitrate dehydrogenase 1/2 (IDH1/2), TP53, and ATRX; codeletion of chromosomes 1p and 19q; DNA methylation; and amplification of genes such as epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor, alpha polypeptide provides a more accurate prognostication and biologic classification than classical histopathological diagnoses, and a number of molecular markers are being incorporated in the new World Health Organization classification of gliomas. However, despite the improved understanding of the molecular subtypes of gliomas and the underlying alterations in specific signaling pathways, these observations have so far failed to result in the successful application of targeted therapies, as has occurred in other solid tumors. To date, the only targeted therapy for gliomas approved by the US Food and Drug Administration is bevacizumab, which targets vascular endothelial growth factor. EGFR remains a dominant molecular alteration in specific glioma subtypes and represents a potentially promising target, with drugs of multiple types targeting EGFR in development including vaccines, antibody drug conjugates, and chimeric antigen receptor (CAR) T cells, despite the prior failures of EGFR tyrosine kinase inhibitors. Immune therapies under investigation include checkpoint inhibitors, vaccines against tumor-associated antigens and tumor-specific antigens, pulsed dendritic cells, heat shock protein-tumor conjugates, and CAR T cells. Mutations in the IDH1/2 genes are central to gliomagenesis in a high proportion of grade II and III gliomas, and ongoing trials are examining vaccines against IDH1, small molecular inhibitors of IDH1 and IDH2, and metabolic components including NAD+ depletion to target IDH-mutated gliomas. The central role of DNA methylation in a subset of gliomas may be targetable, but better understanding of the relation between epigenetic alterations and resulting tumor biology appears necessary. Ultimately, given the prior failure of single-agent targeted therapy in high-grade gliomas, it appears that novel combinatorial therapy or targeted drugs with immunomodulatory or epigenetic approaches will likely be necessary to successfully combat these challenging tumors.
Similar content being viewed by others
Abbreviations
- ATRX:
-
Alpha thalassemia/mental retardation syndrome X-linked
- CDKN2A/B:
-
Cyclin-dependent kinase inhibitor 2A or 2B
- CIC:
-
Capicua transcriptional repressor
- DAXX:
-
Death domain-associated protein
- Epha2:
-
Ephrin type-A receptor 2 precursor
- FUBP1:
-
Far upstream element binding protein 1
- H3F3A:
-
H3 histone, family 3A
- IL-13Ra2:
-
Interleukin 13 receptor subunit alpha 2
- MGMT:
-
0-6-methylguanine-DNA methyltransferase
- MTOR:
-
Mechanistic target of rapamycin
- NF-kB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PARP:
-
Poly ADP ribose polymerase
- PIK3CA:
-
Phosphatidylinositol-4,5-bisphosphonate 3-kinase, catalytic subunit alpha
- PTEN:
-
Phosphatase and tensin homolog
- RB1:
-
Retinoblastoma 1 gene
- STAT3:
-
Signal transducer and activator of transcription 3
- TERT:
-
Telomerase reverse transcriptase
- TP53:
-
Tumor protein 53
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96.
Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–43. Recent phase III trial that demonstrated improvement in progression-free and overall survival in patients treated with tumor treated fields in addition to radiation and adjuvant temozolomide versus radiation and temozolomide alone.
Ostrom QT, Gittleman H, de Blank PM, et al. American Brain Tumor Association Adolescent and Young Adult Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro-Oncology. 2016;18 Suppl 1:i1–50.
Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66.
Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109.
Brat DJ, Verhaak RG, Aldape KD, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–98. Comprehensive, multiplatform analysis dividing lower grade gliomas into three distinct molecular classes that were more concordant with IDH mutation, 1p19q loss, and TP53 alteration than with histology. These delineated groups more accurately predict clinical outcomes and help set the stage for molecular - based definition of these tumors in clinical trials and clinical practice.
Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–508.
Ceccarelli M, Barthel FP, Malta TM, et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–63.
Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8.
Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–77.
Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24(18):2707–14.
Cairncross JG, Wang M, Jenkins RB, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol. 2014;32(8):783–90.
Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–37.
Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–8.
Lee EQ, Kuhn J, Lamborn KR, et al. Phase I/II study of sorafenib in combination with temsirolimus for recurrent glioblastoma or gliosarcoma: North American Brain Tumor Consortium study 05–02. Neuro-Oncology. 2012;14(12):1511–8.
Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333(2):328–35.
Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708.
Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–22.
Sandmann T, Bourgon R, Garcia J, et al. Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: retrospective analysis of the AVAglio trial. J Clin Oncol. 2015;33(25):2735–44.
Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–53.
Wick W, Brandes A, Gorlia T, et al. LB-05PHASE III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the EORTC 26101 trial. Neuro-Oncology. 2015;17 suppl 5:v1.
Swartz AM, Li QJ, Sampson JH. Rindopepimut: a promising immunotherapeutic for the treatment of glioblastoma multiforme. Immunotherapy. 2014;6(6):679–90.
Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353(19):2012–24.
Haas-Kogan DA, Prados MD, Tihan T, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97(12):880–7.
Reardon DA, Wen PY, Mellinghoff IK. Targeted molecular therapies against epidermal growth factor receptor: past experiences and challenges. Neuro-Oncology. 2014;16 Suppl 8:viii7–13.
Prados MD, Byron SA, Tran NL, et al. Toward precision medicine in glioblastoma: the promise and the challenges. Neuro-Oncology. 2015;17(8):1051–63.
Reardon D, Desjardins A, Schuster J, et al. ReACT: long-term survival from a randomized phase II study of rindopepimut (CDX-110) plus bevacizumab in relapsed glioblastoma. Neuro-Oncoloogy. 2015;17 suppl 5:v109.
Inman S. Rindopepimut misses OS endpoint in phase III glioblastoma trial. OncLive. 2016. http://www.onclive.com/web-exclusives/rindopepimut-misses-os-endpoint-in-phase-iii-glioblastoma-trial. Accessed June 10, 2016.
Reilly EB, Phillips AC, Boghaert ER, et al. ABT-414, an antibody drug conjugate targeting a tumor-selective EGFR epitope. Mol Cancer Ther. 2016;15(4):661–9.
Gan H, Kumthekar P, Lassman A, et al. ABT-414 mono- or combination therapy with temozolomide (TMZ) rechallenge in patients with recurrent glioblastoma (GBM) and amplified epidermal growth factor receptor (EGFR): a phase I study. Neuro-Oncology. 2015;17 Suppl 5:v10.
Sampson JH, Mitchell DA. Vaccination strategies for neuro-oncology. Neuro-Oncology. 2015;17 Suppl 7:vii15–25.
Schumacher T, Bunse L, Pusch S, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512(7514):324–7.
Bregy A, Wong TM, Shah AH, Goldberg JM, Komotar RJ. Active immunotherapy using dendritic cells in the treatment of glioblastoma multiforme. Cancer Treat Rev. 2013;39(8):891–907.
Ampie L, Choy W, Lamano JB, Fakurnejad S, Bloch O, Parsa AT. Heat shock protein vaccines against glioblastoma: from bench to bedside. J Neuro-Oncol. 2015;123(3):441–8.
Thaci B, Brown CE, Binello E, Werbaneth K, Sampath P, Sengupta S. Significance of interleukin-13 receptor alpha 2-targeted glioblastoma therapy. Neuro-Oncology. 2014;16(10):1304–12.
Johnson LA, Scholler J, Ohkuri T, et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med. 2015;7(275):275ra222.
Tronnier M, Mitteldorf C. Treating advanced melanoma: current insights and opportunities. Cancer Manag Res. 2014;6:349–56.
Wakimoto H, Tanaka S, Curry WT, et al. Targetable signaling pathway mutations are associated with malignant phenotype in IDH-mutant gliomas. Clin Cancer Res. 2014;20(11):2898–909.
Chen R, Ravindra VM, Cohen AL, et al. Molecular features assisting in diagnosis, surgery, and treatment decision making in low-grade gliomas. Neurosurg Focus. 2015;38(3):E2.
Tateishi K, Wakimoto H, Iafrate AJ, et al. Extreme vulnerability of IDH1 mutant cancers to NAD+ depletion. Cancer Cell. 2015;28(6):773–84.
Duke Comprehensive Cancer Center, Duke University. Patients with IDH1 positive recurrent grade II glioma enrolled in a safety and immunogenicity study of tumor-specific peptide vaccine. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 2000. https://clinicaltrials.gov/ct2/show/NCT02193347. Accessed March 23, 2016.
National Center for Tumor Diseases, Heidelberg. Targeting IDH1R132H in WHO Grade III-IV IDH1R132H-mutated gliomas by a peptide vaccine—a phase I safety, tolerability and immunogenicity multicenter trial (NOA-16). In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 2000. https://clinicaltrials.gov/ct2/show/NCT02454634. Accessed March 23, 2016.
Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483(7390):479–83.
Flavahan WA, Drier Y, Liau BB, et al. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature. 2016;529(7584):110–4.
Selby GB, Upchurch C, Townsend J, Eyre HJ. A phase II evaluation of fazarabine in high-grade gliomas: a Southwest Oncology Group study. Cancer Chemother Pharmacol. 1994;34(2):179–80.
Rheinbay E, Louis DN, Bernstein BE, Suva ML. A tell-tail sign of chromatin: histone mutations drive pediatric glioblastoma. Cancer Cell. 2012;21(3):329–31.
Majuelos-Melguizo J, Rodriguez MI, Lopez-Jimenez L, et al. PARP targeting counteracts gliomagenesis through induction of mitotic catastrophe and aggravation of deficiency in homologous recombination in PTEN-mutant glioma. Oncotarget. 2015;6(7):4790–803.
Gray GK, McFarland BC, Nozell SE, Benveniste EN. NF-kappaB and STAT3 in glioblastoma: therapeutic targets coming of age. Expert Rev Neurother. 2014;14(11):1293–306.
Wen PY, Chang SM, Lamborn KR, et al. Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas: North American Brain Tumor Consortium trial 04–02. Neuro-Oncology. 2014;16(4):567–78.
Chinnaiyan P, Won M, Wen PY, et al. RTOG 0913: a phase 1 study of daily everolimus (RAD001) in combination with radiation therapy and temozolomide in patients with newly diagnosed glioblastoma. Int J Radiat Oncol Biol Phys. 2013;86(5):880–4.
Mrugala MM, Engelhard HH, Dinh Tran D, et al. Clinical practice experience with NovoTTF-100A system for glioblastoma: the Patient Registry Dataset (PRiDe). Semin Oncol. 2014;41 Suppl 6:S4–13.
Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–93. Study of the clonal evolution of tumor recurrences in low-grade gliomas demonstrating that the sequential recurrences of a tumor may derive phylogenetically from different earlier evolutionary stages of the initial tumor and therefore contain divergent driver mutations that need to be treated differently. This study elucidates one of the great challenges of targeted therapies and may explain one of the reasons single-agent targeted therapies have failed to demonstrate efficacy to date in these tumors.
van Thuijl HF, Mazor T, Johnson BE, et al. Evolution of DNA repair defects during malignant progression of low-grade gliomas after temozolomide treatment. Acta Neuropathol. 2015;129(4):597–607.
Acknowledgments
We thank Kristin Kraus, M.Sc., for editorial assistance in preparing this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ricky Chen declares that he has no conflict of interest.
Adam L. Cohen declares that he has no conflict of interest.
Howard Colman is the site PI for clinical trials (institutional contract) conducted with Plexxikon and NewLink Genetics and has received compensation from Roche, Genentech, Upsher-Smith, Oxigene, CytRx, Novocure, and Omniox for service on advisory boards.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Neuro-oncology
Rights and permissions
About this article
Cite this article
Chen, R., Cohen, A.L. & Colman, H. Targeted Therapeutics in Patients With High-Grade Gliomas: Past, Present, and Future. Curr. Treat. Options in Oncol. 17, 42 (2016). https://doi.org/10.1007/s11864-016-0418-0
Published:
DOI: https://doi.org/10.1007/s11864-016-0418-0