Abstract
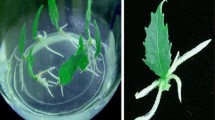
Poplar 741 [Populus alba × (P. davidiana + P. simonii) × P. tomentosa] leaves were rooted within 8 days when cultured on 1/2 MS medium. The spatial distribution of endogenous indole-3-acetic acid (IAA) and its dynamic changes in the rhizogenesis were investigated, using an immunohistochemical approach. Anatomical analyses showed that the root primordia arose from vascular cambium cells in the basal regions of the petioles of the leaves. Before root induction, immunostaining patterns showed a basipetally decreasing gradient of IAA along the leaves. Three days after induction, the IAA immunostaining pattern observed along the leaves was high at both ends and low in the middle. And IAA in the basal regions of the petiole was distributed mainly in the vascular bundles. Localized application of 2,3,5-triiodobenzoic acid (TIBA) on laminas of the leaves delayed the accumulation of IAA in the vascular bundles of the basal regions of the petioles, but not in the mesophyll of the laminas. These data indicate that an accumulation of IAA in the vascular bundles of the basal regions of the petioles induces the occurrence of rhizogenesis of poplar leaves. And IAA accumulated in the vascular bundle of the basal region of the petiole results from its polar transportation from mesophyll of the laminas, rather than by in situ IAA generation.







Similar content being viewed by others
References
Avsian-Kretchmer O, Cheng JC, Chen L, Moctezuma E, Sung ZR (2002) Indole acetic acid distribution coincides with vascular differentiation pattern during Arabidopsis leaf ontogeny. Plant Physiol 130:199–209
Blakesley D (1994) Auxin metabolism and adventitious root initiation. In: Davis TD, Haissig BE (eds) Biology of adventitious root formation. Plenum, New York, pp 143–154
Chen D, Ren Y, Deng Y, Zhao J (2010) Auxin polar transport is essential for the development of zygote and embryo in Nicotiana tabacum L. and correlated with ABP1 and PM H+-ATPase activities. J Exp Bot 61:1853–1867
Cooper WC (1935) Hormones in relation to root formation on stem cuttings. Plant Physiol 10:789–794
Falasca G, Zaghi D, Possenti M, Altamura MM (2004) Adventitious root formation in Arabidopsis thaliana thin cell layers. Plant Cell Rep 23:17–25
Gangopadhyay M, Chakraborty D, Dewanjee S, Bhattacharya S (2010) Clonal propagation of Zephyranthes grandiflora using bulbs as explants. Biol Plant 54:793–797
Gaspar T, Kevers C, Hausman JF, Ripetti V (1994) Peroxidase activity and endogenous free auxin during adventitious root formation. In: Lumdsen PJ, Nicholas JR, Davies WJ (eds) Physiology growth and development of plants in culture. Kluwer, Dordrecht, pp 289–298
Geldner N, Friml J, Stierhof YD, Jurgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413:425–428
Heloir MC, Kevers C, Hausman JF, Gaspar T (1996) Changes in the concentrations of auxins and polyamines during rooting of in-vitro-propagated walnut shoots. Tree Physiol 16:515–519
Holgate CS, Jackson P, Cowen PN, Bird CC (1983) Immunogold-silver staining: new method of immunostaining with enhanced sensitivity. J Histochem Cytochem 31:938–944
Hou ZX, Huang WD (2005) Immunohistochemical localization of IAA and ABP1 in strawberry shoot apexes during floral induction. Planta 222:678–687
Li SW, Xue LG, Xu SJ, Feng HY, An LZ (2009) Hydrogen peroxide acts as a signal molecule in the adventitious root formation of mung bean seedlings. Environ Exp Bot 65:63–71
Li YH, Chen QZ, Xiao NJ, Chen YF, Li XJ, Staehelin C, Huang XL (2008) Characteristics of adventitious root formation in cotyledon segments of mango (Mangifera indica L cv. Zihua): two induction patterns, histological origins and the relationship with polar auxin transport. Plant Growth Regul 54:165–177
Ljung K, Bhalerao RP, Sandberg G (2001) Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J 28:465–474
Ludwig-Muller J, Vertocnik A, Town CD (2005) Analysis of indole-3-butyric acid-induced adventitious root formation on Arabidopsis stem segments. J Exp Bot 56:2095–2105
Mertens R, Eberle J, Arnscheidt A, Ledebur A, Weiler EW (1985) Monoclonal antibodies to plant growth regulators. II. Indole-3-acetic acid. Planta 166:389–393
Moctezuma E (1999) Changes in auxin patterns in developing gynophores of the peanut plant (Arachis hypogaea L.). Ann Bot 83:235–242
Negi S, Sukumar P, Liu X, Cohen JD, Muday GK (2010) Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant J 61:3–15
Nourissier S, Monteuuis O (2008) In vitro rooting of two Eucalyptus urophylla × Eucalyptus grandis mature clones. In Vitro Cell Dev Biol Plant 44:263–272
Perbal G, Leroux Y, Driss-Ecole D (1982) Mise en evidence de I’AIA-5–3H par autoradiographie dans le coleoptile de Bie. Physiol Plant 54:167–173
Ramirez-Carvajal GA, Morse AM, Dervinis C, Davis JM (2009) The cytokinin type-B response regulator PtRR13 is a negative regulator of adventitious root development in Populus. Plant Physiol 150:759–771
Thomas C, Bronner R, Molinier J, Prinsen E, van Onckelen H, Hahne G (2002) Immuno-cytochemical localization of indole-3-acetic acid during induction of somatic embryogenesis in cultured sunflower embryos. Planta 215:577–583
Vieten A, Sauer M, Brewer PB, Friml J (2007) Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci 12:160–168
Xu M, Zhu L, Shou H, Wu P (2005) A PIN1 family gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiol 46:1674–1681
Xuan W, Zhu FY, Xu S, Huang BK, Ling TF, Qi JY, Ye MB, Shen WB (2008) The heme oxygenase/carbon monoxide system is involved in the auxin-induced cucumber adventitious rooting process. Plant Physiol 148:881–893
Zhao Y (2008) The role of local biosynthesis of auxin and cytokinin in plant development. Curr Opin Plant Biol 11:16–22
Zhong R, Thompson J, Ottesen E, Lamppa GK (2010) A forward genetic screen to explore chloroplast protein import in vivo identifies Moco sulfurase, pivotal for ABA and IAA biosynthesis and purine turnover. Plant J 63:44–59
Zhou DX, Yin K, Xu ZH, Xue HW (2003) Effect of polar auxin transport on rice root development. Acta Bot Sin 45:1421–1427
Acknowledgments
We sincerely thanked Dr Mingyong Chen (College of Veterinary Medicine, China Agricultural University, China) and Dr Zhixia Hou (School of Forestry, Beijing Forestry University, China) for their kind help with experimental methods and the equipment. This work was supported by the National Natural Science Foundation of China (Grant No. 31171933).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, N., Pei, D. & Yin, W. Tissue-specific localization and dynamic changes of endogenous IAA during poplar leaf rhizogenesis revealed by in situ immunohistochemistry. Plant Biotechnol Rep 6, 165–174 (2012). https://doi.org/10.1007/s11816-011-0209-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-011-0209-9