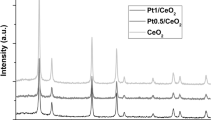
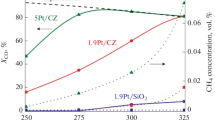
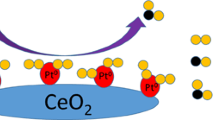
Abstract
A comparison study was performed of the water-gas shift (WGS) reaction over Pt and ceria-promoted Pt catalysts supported on CeO2, ZrO2, and TiO2 under rather severe reaction conditions: 6.7 mol% CO, 6.7 mol% CO2, and 33.2 mol% H2O in H2. Several techniques—CO chemisorption, temperature-programmed reduction (TPR), and inductively coupled plasma-atomic emission spectroscopy (ICP-AES)—were employed to characterize the catalysts. The WGS reaction rate increased with increasing amount of chemisorbed CO over Pt/ZrO2, Pt/TiO2, and Pt-CeO x /ZrO2, whereas no such correlation was found over Pt/CeO2, Pt-CeO x /CeO2, and Pt-CeO x /TiO2. For these catalysts in the absence of any impurities such as Na+, the WGS activity increased with increasing surface area of the support, showed a maximum value, and then decreased as the surface area of the support was further increased. An adverse effect of Na+ on the amount of chemisorbed CO and the WGS activity was observed over Pt/CeO2. Pt-CeO x /TiO2 (51) showed the highest WGS activity among the tested supported Pt and Pt-CeOx catalysts. The close contact between Pt and the support or between Pt and CeO x , as monitored by H2-TPR, is closely related to the WGS activity. The catalytic stability at 583K improved with increasing surface area of the support over the CeO2- and ZrO2-supported Pt and Pt-CeO x catalysts.
Similar content being viewed by others
References
M. I. Temkin, Adv. Catal., 28, 173 (1979).
D. S. Newsome, Catal. Rev. Sci. Eng., 21, 275 (1980).
O. Ilinich, W. Ruettinger, X. S. Liu and R. Ferrauto, J. Catal., 247, 112 (2007).
T. Giroux, S. Hwang, Y. Liu, W. Ruettinger and L. Shore, Appl. Catal. B: Environ., 56, 95 (2005).
P. Panagiotopoulou and D. I. Kondarides, Catal. Today, 112, 49 (2006).
X.H. Liu, W. Ruettinger, X. Xu and R. Farrauto, Appl. Catal. B: Environ., 56, 69 (2005).
H.N. Evin, G. Jacobs, J. Ruiz-Martinez, G.A. Thomas and B.H. Davis, Catal. Lett., 120, 166 (2008).
W. L. Deng and M. Flytzani-Stephanopoulos, Angew. Chem., 118, 2343 (2006).
D. Tibiletti, F. C. Meunier, A. Goguet, D. Reid, R. Burch, M. Boaro, M. Vicario and A. Trovarelli, J. Catal., 244, 183 (2006).
S. Ricote, G. Jacobs, M. Milling, Y. Ji, P.M. Patterson, B. H. Davis, Appl. Catal. A: Gen., 303, 35 (2006).
H.C. Lee, D.H. Lee, O.Y. Lim, S. H. Kim, Y. T. Kim, E.Y. Ko and E. D. Park, Stud. Surf. Sci. Catal., 167, 201 (2007).
R. Radhakrishnan, R. R. Willigan, Z. Dardas and T.H. Vanderspurt, AIChE J., 52, 1888 (2006).
M. Laniecki and M. Ignacik, Catal. Today, 116, 400 (2006).
X. Wang, R. J. Gorte and J. P. Wagner, J. Catal., 212, 225 (2002).
J.M. Zalc, V. Sokolovskii and D. G. Löffler, J. Catal., 206, 169 (2002).
H. Iida and A. Igarashi, Appl. Catal. A: Gen., 298, 152 (2006).
P. Panagiotopoulou, A. Christodoulakis, D. I. Kondarides and S. Boghosian, J. Catal., 240, 114 (2006).
P. Panagiotopoulou and D. I. Kondarides, J. Catal., 267, 57 (2009).
K.G. Azzam, I.V. Babich, K. Seshan and L. Lefferts, Appl. Catal. A: Gen., 338, 66 (2008).
Y. T. Kim, E. D. Park, H. C. Lee, D. Lee and K. H. Lee, Appl. Catal. B: Environ., 90, 45 (2009).
X. Zhu, T. Hoang, L. L. Lobban and R.G. Mallinson, Catal. Lett., 129, 135 (2009).
I.D. González, R.M. Navarro, W. Wen, N. Marinkovic, J.A. Rodriguéz, F. Rosa and J. L.G. Fierro, Catal. Today, 149, 372 (2010).
H. C. Yao and Y. F. Yu Yao, J. Catal., 86, 254 (1984).
C.-P. Hwang and C.-T. Yeh, J. Mol. Catal. A: Chem., 112, 295 (1996).
R.W. McCabe, C. Wong and H. S. Woo, J. Catal., 114, 354 (1988).
A. Holmgren and B. Andersson, J. Catal., 178, 14 (1998).
C. Li, Y. Sakata, T. Arai, K. Domen, K. I. Maruya and T. Onishi, J. Chem. Soc. Faraday Trans.1, 85, 929 (1989).
R. Srinivasan, M.B. Harris, S. F. Simpson and B.H. Davis, J. Mater. Res., 3, 787 (1988).
R. A. Spurr and H. Myers, Anal. Chem., 59, 761 (1957).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, Y.T., Park, E.D. Water-gas shift reaction over supported Pt and Pt-CeOx catalysts. Korean J. Chem. Eng. 27, 1123–1131 (2010). https://doi.org/10.1007/s11814-010-0210-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-010-0210-y