Abstract
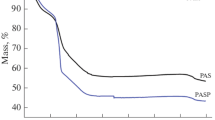
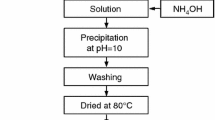
The precursor of ammonium aluminum carbonate hydroxide was synthesized by using aluminum sulfate (Al2(SO4)3) and ammonium carbonate ((NH4)2CO3). The effects of α-Al2O3 seeds and mixture composed of α-Al2O3 and ammonium nitrate, as well as multiplex catalysts (AT) on phase transformation of alumina in sintering process were investigated respectively. The results show that the α-Al2O3 seeds and the mixture of α-Al2O3 and ammonium nitrate can lower the phase transformation temperature of α-Al2O3 to different extents while the particles obtained agglomerate heavily. AT has great potential synergistic effects on the phase transformation of alumina and reduces the phase transformation temperature of α-Al2O3 and the trends of necking-formation between particles. Therefore the dispersion of powder particles is improved significantly.
Similar content being viewed by others
References
ZHANG Mei-ge. Development of the methods to make the high purity alumina [J]. Functional Material, 1993, 24(2): 187–191. (in Chinese)
WU Zhi-hong. Preparation of nanoparticle alumina and its application in catalysis [J]. Industrial Catalysis, 2004, 12(2): 35–39. (in Chinese)
ZHANG Ai-fei, LIU Ji-ping. A new-precipitation method for the preparation of alumina nanopowders [J]. Inorganic Chemicals Industry, 2003, 35(2): 27–28. (in Chinese)
LIN Yuan-hua, ZHANG Zhong-tai, HUANG Chuan-yong, et al. Preparation of high purity and ultra-fine α-Al2O3 powder by pyrolysis NH4AlO(OH)HCO3[J]. Journal of the Chinese Ceramic Society, 2000, 28(3): 268–271. (in Chinese)
Morinaga K, Torikai T, Nakagawa K, et al. Fabrication of fine α-alumina powder by thermal decomposition of ammonium alumina carbonate hydroxide (AACH) [J]. Acta Mater, 2000, 48(18–19): 4735–4741.
Levin I, Brandon D. Metastable alumina polymorphs: crystal structures and transition sequences[J]. J Am Ceram Soc, 1998, 81(8): 1995–2012.
WU Yu-cheng, SONG Zhen-ya, YANG Ye, et al. Mechanism and control of α-phase transformation of alumina[J]. Chinese Journal of Rare Metals, 2004, 28(6): 1043–1048. (in Chinese)
Dynys F W, Halloran J W. Alpha alumina formation in alum-derived gamma alumina[J]. J Am Ceram Soc, 1982, 65(9): 442–448.
Bagwell R B, Messing G L. Effect of seeding and water vapor on the nucleation and growth of α-Al2O3 from γ-Al2O3[J]. J Am Ceram Soc, 1999, 82(4): 825–832.
Oh Ch S, Tomandl G, Lee M H, et al. Effect of an added seed on the phase transformation and the powder properties in the fabrication of Al2O3 powder by the sol-gel process[J]. J Mater Sci, 1996, 31(20): 5321–5325.
YANG Ye, WU Yu-cheng, LI Yong, et al. Preparation of ultrafine α-Al2O3 powder by thermal decomposition of AACH at low temperature[J]. The Chinese Journal of Process Engineering, 2002, 2(4): 325–329. (in Chinese)
Kumagai M, Messing G. Controlled transformation and sintering of a boehmite sol-gel by α-alumina seeding[J]. J Am Ceram Soc, 1985, 68(9): 500–505.
ZENG Wen-ming, CHEN Nian-yi, GUI Lin-hua, et al. Synthesis of nanopowers using inorganic salt and its physical chemistry[J]. Journal of Inorganic Materials, 1998, 13(6): 887–892. (in Chinese)
Yen F S, Wen H L, Hsu Y T. Crystallite size growth and the derived dilatometric effect during θ- to α- phase transformation on nano-sized alumina poweders[J]. J Crystal Growth, 2001, 233 (4): 761–773.
Rajendran S. Production of ultrafine alumina powder and fabrication of fine grained strong ceramics[J]. J Mater Sci, 1994, 29(21): 5664–5672.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, J., Deng, H., Wan, Y. et al. Preparation of ultrafine α-Al2O3 powders by catalytic sintering of ammonium aluminum carbonate hydroxide at low temperature. J Cent. South Univ. Technol. 13, 367–372 (2006). https://doi.org/10.1007/s11771-006-0050-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11771-006-0050-4