Abstract
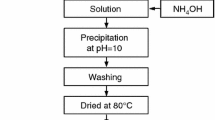
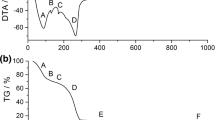
Fine alpha-alumina (α-Al2O3) powder was prepared by simple precipitation technique using aluminium salt and ammonia as a precipitating agent. In this method, an aqueous solution of aluminium nitrate and ammonia were mixed with continuous stirring both in the presence and in the absence of polyethylene glycol (PEG). A controlled pH range of 7.5–8.5 was maintained throughout the synthesis process. A comparative study between the alumina powder prepared in the presence and absence of PEG was drawn into attention. The washing of the precipitate was deliberately avoided in one case to study the effect of washing on phase formation temperature. The synthesized powders were calcined at different temperatures and characterized by X-ray diffraction study, thermal analysis, infrared analysis, microstructural study, and particle size analysis. The results showed less agglomerated fine alumina powder formation in its pure α-Al2O3 phase at 1050°C in the presence of PEG.









Similar content being viewed by others
REFERENCES
Behera, P.S., Sarkar, R., and Bhattacharyya, S., Nano alumina: A review of the powder synthesis method, Interceram, 2016, vol. 65, nos. 1–2, pp. 10–16.
Souza Santos, P., Souza Santos, H., and Toledo, S.P., Standard transition aluminas. Electron microscopy studies, Mater. Res., 2000, vol. 3, no. 4, pp. 104–114.
Kaya, C., Kaya, F., Boccaccini, A.R., and Chawla, K.K., Fabrication and characterisation of Ni-coated carbon fibre-reinforced alumina ceramic matrix composites using electrophoretic deposition, Acta Mater., 2001, vol. 49, no. 7, pp. 1189–1197.
Huang, C.L., Wang, J.J., and Huang, C.Y., Sintering behavior and microwave dielectric properties of nano alpha-alumina, Mater. Lett., 2005, vol. 59, no. 28, pp. 3746–3749.
Szabo, N., Lee, C., Trimboli, J., Figueroa, O., Ramamoorthy, R., Midlam-Mohler, S., Soliman, A., Verweij, H., Dutta, P., and Akbar, S., Ceramic-based chemical sensors, probes and field-tests in automobile engines, J. Mater. Sci., 2003, vol. 38, no. 21, pp. 4239–4245.
Elsen, S.R. and Ramesh, T., Optimization to develop multiple response hardness and compressive strength of zirconia reinforced alumina by using RSM and GRA, Int. J. Refract. Met. Hard Mater., 2015, vol. 52, pp. 159–164.
Pathak, L.C., Singh, T.B., Das, S., Verma, A.K., and Ramachandrarao, P., Effect of pH on the combustion synthesis of nano-crystalline alumina powder, Mater. Lett., 2002, vol. 57, no. 2, pp. 380–385.
Laine, R.M., Marchal, J.C., Sun, H.P., and Pan, X.Q., Nano-α-Al2O3 by liquid-feed flame spray pyrolysis, Nat. Mater., 2006, vol. 5, no. 9, pp. 710–712.
Al’myasheva, O.V., Korytkova, E.N., Maslov, A.V., and Gusarov, V.V., Preparation of nanocrystalline alumina under hydrothermal conditions, Inorg. Mater., 2005, vol. 41, no. 5, pp. 460–467.
Zhuravlev, V.D., Vasil’ev, V.G., Vladimirova, E.V., Shevchenko, V.G., Grigorov, I.G., Bamburov, V.G., Beketov, A.R., and Baranov, M.V., Glycine-nitrate combustion synthesis of finely dispersed alumina, Glass Phys. Chem., 2010, vol. 36, no. 4, pp. 506–512.
Kim, S.M., Lee, Y.J., Jun, K.W., Park, J.Y., and Potdar, H.S., Synthesis of thermo-stable high surface area alumina powder from sol–gel derived boehmite, Mater. Chem. Phys., 2007, vol. 104, no. 1, pp. 56–61.
Tok, A.I.Y., Boey, F.Y.C., and Zhao, X.L., Novel synthesis of Al2O3 nano-particles by flame spray pyrolysis, J. Mater. Process. Technol., 2006, vol. 178, nos. 1–3, pp. 270–273.
Ganesh, I., Torres, P.M., and Ferreira, J.M.F., Densification ability of combustion-derived Al2O3 powders, Ceram. Int., 2009, vol. 35, no. 3, pp. 1173–1179.
Wu, Z., Shen, Y., Dong, Y., and Jiang, J., Study on the morphology of α-Al2O3 precursor prepared by precipitation method, J. Alloys Compd., 2009, vol. 467, nos. 1–2, pp. 600–604.
Kano, J., Saeki, S., Saito, F., Tanjo, M., and Yamazaki, S., Application of dry grinding to reduction in transformation temperature of aluminum hydroxides, Int. J. Miner. Process., 2000, vol. 60, no. 2, pp. 91–100.
Shiau, F.S. and Fang, T.T., Low-temperature synthesis of α-alumina using citrate process with α-alumina seeding, Mater. Chem. Phys., 1999, vol. 60, no. 1, pp. 91–94.
Kim, H.J., Kim, T.G., Kim, J.J., Park, S.S., Hong, S.S., and Lee, G.D., Influences of precursor and additive on the morphology of nanocrystalline α-alumina, J. Phys. Chem. Solids, 2008, vol. 69, nos. 5–6, pp. 1521–1524.
Wu, Y.Q., Zhang, Y.F., Huang, X.X., and Guo, J.K., Preparation of plate like nano alpha alumina particles, Ceram. Int., 2001, vol. 27, no. 3, pp. 265–268.
Lee, J.S., Kim, H.S., Park, N.K., Lee, T.J., and Kang, M., Low temperature synthesis of α-alumina from aluminum hydroxide hydrothermally synthesized using [Al (C2O4)x(OH)y] complexes, Chem. Eng. J., 2013, vol. 230, pp. 351–360.
Temuujin, J., Jadambaa, T., Mackenzie, K.J.D., Angerer, P., Porte, F., and Riley, F., Thermal formation of corundum from aluminium hydroxides prepared from various aluminium salts, Bull. Mater. Sci., 2000, vol. 23, no. 4, pp. 301–304.
Shin, D.C., Park, S.S., Kim, J.H., Hong, S.S., Park, J.M., Lee, S.H., Kim, D.S., and Lee, G.D., Study on α-alumina precursors prepared using different ammonium salt precipitants, J. Ind. Eng. Chem., 2014, vol. 20, no. 4, pp. 1269–1275.
Parida, K.M., Pradhan, A.C., Das, J., and Sahu, N., Synthesis and characterization of nano-sized porous gamma-alumina by control precipitation method, Mater. Chem. Phys., 2009, vol. 113, no. 1, pp. 244–248.
Potdar, H.S., Jun, K.W., Bae, J.W., Kim, S.M., and Lee, Y.J., Synthesis of nano-sized porous γ-alumina powder via a precipitation/digestion route, Appl. Catal., A, 2007, vol. 321, no. 2, pp. 109–116.
Zhao, R., Guo, F., Hu, Y., and Zhao, H., Self-assembly synthesis of organized mesoporous alumina by precipitation method in aqueous solution, Microporous Mesoporous Mater., 2006, vol. 93, nos. 1–3, pp. 212–216.
Sun, X., Li, J., Zhang, F., Qin, X., Xiu, Z., Ru, H., and You, J., Synthesis of nanocrystalline α-Al2O3 powders from nanometric ammonium aluminum carbonate hydroxide, J. Am. Ceram. Soc., 2003, vol. 86, no. 8, pp. 1321–1325.
Sun, Z.X., Zheng, T.T., Bo, Q.B., Vaughan, D., and Warren, M., Effects of alkali metal ions on the formation of mesoporous alumina, J. Mater. Chem., 2008, vol. 18, no. 48, pp. 5941–5947.
Li, J.G. and Sun, X., Synthesis and sintering behavior of a nanocrystalline α-alumina powder, Acta Mater., 2000, vol. 48, no. 12, pp. 3103–3112.
Wu, Y.Q., Zhang, Y.F., Huang, X.X., and Guo, J.K., Preparation of plate like nano alpha alumina particles, Ceram. Int., 2001, vol. 27, no. 3, pp. 265–268.
Kou, Y., Wang, S., Luo, J., Sun, K., Zhang, J., Tan, Z., and Shi, Q., Thermal analysis and heat capacity study of polyethylene glycol (PEG) phase change materials for thermal energy storage applications, J. Chem. Thermodyn., 2019, vol. 128, pp. 259–274.
Dilshad, M.R., Islam, A., Hamidullah, U., Jamshaid, F., Ahmad, A., Butt, M.T.Z. and Ijaz, A., Effect of alumina on the performance and characterization of cross-linked PVA/PEG 600 blended membranes for CO2/N2 separation, Sep. Purif. Technol., 2019, vol. 210, pp. 627–635.
Gitzen, W.H., Alumina as a Ceramic Material, Hoboken: Wiley, 1970.
Bhattacharyya, S. and Behera, P.S., Synthesis and characterization of nano-sized α-alumina powder from kaolin by acid leaching process, Appl. Clay Sci., 2017, vol. 146, pp. 286–290.
Kim, H.J., Kim, T.G., Kim, J.J., Park, S.S., Hong, S.S., and Lee, G.D., Influences of precursor and additive on the morphology of nanocrystalline α-alumina, J. Phys. Chem. Solids, 2008, vol. 69, nos. 5–6, pp. 1521–1524.
Mokoena, T.P., Linganiso, E.C., Swart, H.C., Kumar, V., and Ntwaeaborwa, O.M., Cooperative luminescence from low temperature synthesized α-Al2O3:Yb3+ phosphor by using solution combustion, Ceram. Int., 2017, vol. 43, no. 1, pp. 174–181.
Wang, S., Li, X., Wang, S., Li, Y., and Zhai, Y., Synthesis of γ-alumina via precipitation in ethanol, Mater. Lett., 2008, vol. 62, no. 20, pp. 3552–3554.
Singh, R. and Bhattacharyya, S., Synthesis of mullite precursor powder in diphasic gel form, Trans. Ind. Ceram. Soc., 2014, vol. 73, no. 2, pp. 98–101.
Behera, P.S., Bhattacharyya, S., and Sarkar, R., Effect of citrate to nitrate ratio on the sol-gel synthesis of nanosized α-Al2O3 powder, Ceram. Int., 2017, vol. 43, no. 17, pp. 15 221–15 226.
ACKNOWLEDGMENTS
Authors would like to thanks the XRD-Texture lab at Department of Metallurgical and Materials Engineering, NIT Rourkela supported by DST-FIST (grant no. SR/FST/ETI-344-/2013 C and G Dated July 7, 2014).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pallavi Suhasinee Behera, Sunipa Bhattacharyya Studies on the Low-Temperature Synthesis of Fine α-Al2O3 Powder by Precipitation Route. Glass Phys Chem 46, 312–320 (2020). https://doi.org/10.1134/S1087659620040033
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659620040033