Abstract
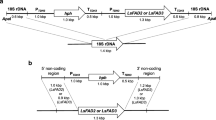
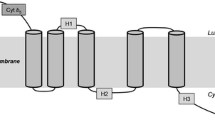
Two cDNA clones with homology to known desaturase genes were isolated from the fungus Mortierella alpina. The open reading frame in one clone encoded 399 amino acids and exhibited Δ12-desaturase activity when expressed in Saccharomyces cerevisiae in the presence of endogenous fatty acid substrate oleic acid. The insert in another clone contained an open reading frame encoding 457 amino acids and exhibited Δ6-desaturase activity in S. cerevisiae in the presence of exogenous fatty acid substrate linoleic acid. Expression of the Δ12-desaturase gene under appropriate media and temperature conditions led to the production of linoleic acid at levels up to 25% of the total fatty acids in yeast. When linoleic acid was provided as an exogenous substrate to the yeast cultures expressing the Δ6-desaturase activity, the level of γ-linolenic acid reached 10% of the total yeast fatty acids. Co-expression of both the Δ6- and Δ12-desaturase cDNA resulted in the endogenous production of γ-linolenic acid. The yields of γ-linolenic acid reached as high as 8% of total fatty acids in yeast.
Similar content being viewed by others
Abbreviations
- GC:
-
gas chromatography
- GLA:
-
γ-linolenic acid
- LC-PUFA:
-
long-chain polyunsaturated fatty acid
- MS:
-
mass spectrometry
- PCR:
-
polymerase chain reaction
- TPI:
-
triose phosphate isomerase
References
Horrobin, D.F. (1992) Nutritional and Medical Importance of Gamma-Linolenic Acid, Prog. Lipid Res. 31, 163–194.
Brenner, R.R. (1976) Regulatory Function of Δ6-Desaturase—A Key Enzyme of Polyunsaturated Fatty Acid Synthesis, Adv. Exp. Med. Biol. 83, 85–101.
Phillips, J.C., and Huang, Y.-S. (1996) Natural Sources and Biosynthesis of γ-Linolenic Acid: An Overview, in γ-Linolenic Acid: Metabolism and Its Roles in Nutrition and Medicine (Huang, Y.-S., and Milles, D.E., eds., AOCS Press, Champaign, pp. 1–13.
Sakamoto, T., Wada, H., Nishida, I., Ohmori, M., and Murata, N. (1994) Identification of Conserved Domains in the Δ12 Desaturases of Cyanobacteria, Plant Mol. Biol. 24, 643–650.
Murata, N., Deshnium, P., and Tasaka, Y. (1996) Biosynthesis of Gamma-Linolenic Acid in the Cyanobacterium Spirulina platensis, in γ-Linolenic Acid: Metabolism and Its Roles in Nutrition and Medicine (Huang, Y.-S., and Milles, D.E., eds.), AOCS Press, Champaign, pp. 22–32.
Okuley, J., Lightner, J., Feldmann, K., Yadav, N., Lark, E., and Browse, J. (1994) Arabidopsis FAD2 Gene Encodes the Enzyme That Is Essential for Polyunsaturated Lipid Synthesis, Plant Cell 6, 147–158.
Heppard, E.P., Kinney, A.J., Stecca, K.L., and Miao, G.-H. (1996) Developmental and Growth Temperature Regulation of Two Different Microsomal ω-6 Desaturase Genes in Soybeans, Plant Physiol. 110, 311–319.
Kirsch, C., Hahlbrock, K., and Somssich, I.E. (1997) Rapid and Transient Induction of a Parsley Microsomal Δ12 Fatty Acid Desaturase mRNA by Fungal Elicitor, Plant Physiol. 115, 283–289.
Reddy, A.S., Nuccio, M.L., Gross, L.M., and Thomas, T.L. (1993) Isolation of a Δ6-Desaturase Gene from the Cyanobacterium Synechocystis sp. Strain PCC6803 by Gain-of-Function Expression in Anabaena sp. Strain PCC7120, Plant Mol. Biol. 27, 293–300.
Sayanova, O., Smith, M.A., Lapinsk, P., Stobart, A.K., Dobson, G., Christie, W.W., Shewry, P.R., and Napier, J.A. (1997) Expression of a Borage Desaturase cDNA Containing an N-Terminal Cytochrome b5 Domain Results in the Accumulation of High Levels of Δ6-Desaturated Fatty Acids in Transgenic Tobacco, Proc. Natl. Acad. Sci. USA 94, 4211–4216.
Napier, J.A., Hey, S.J., Lacey, D.J., and Shewry, P.R. (1998) Identification of a Caenorhabditis elegans Δ6-Fatty-Acid-Desaturase by Heterologous Expression in Saccharomyces cerevisiae, Biochem. J. 330, 611–614.
Arondel, V., Lemieux, B., Hwang, I., Gibson, S., Goodman, H., and Somerville, C.R. (1992) Map-Based Cloning of a Gene Controlling Omega-3 Fatty Acid Desaturation in Arabidoposis, Science 258, 1353–1355.
Yadav, N.S., Wierzbicki, A., Aegerter, M., Caster, C.S., Perez-Grau, L., Kinney, A.J., Hitz, W.D., Booth, R., Schweiger, B., Stecca, K.L., Allen, S.M., Blackwell, M., Reiter, R.S., Carlson, T.J., Russell, S.H., Feldmann, K.A., Pierce, J., and Browse, J. (1993) Cloning of Higher Plant ω-3 Fatty Acid Desaturases, Plant Physiol. 103, 467–476.
Shanklin, J., White, E., and Fox, B.G. (1994) Eight Histidine Residues Are Catalytically Essential in a Membrane-Associated Iron Enzyme, Stearoyl-CoA Desaturase, and Are Conserved in Alkane Hydroxylase and Xylene Monooxygenase, Biochemistry 33, 12787–12794.
Knutzon, D.S., Thurmond, J.A., Huang, Y.-S., Chaudhary, S., Bobik, E.G., Jr., Chan, G.M., Kirchner, S.J., and Mukerji, P. (1998) Identification of Δ5-Desaturase from Mortierella alpina by Heterologous Expression in Baker’s Yeast and Canola, J. Biol. Chem. 273, 29360–29366.
Michaelson, L.V., Lazarus, C.M., Griffiths, G., Napier, J.A., and Stobart, A.K. (1998) Isolation of a Δ5-Fatty Acid Desaturase Gene from Mortierella alpina, J. Biol. Chem. 273, 19055–19059.
van de Loo, F.N., Broun, P., Turner, S., and Somerville, C.R. (1995) An Oleate 12-Hydroxylase from Ricinus communis L. Is a Fatty Acyl Desaturase Homolog, Proc. Natl. Acad. Sci. USA 92, 6743–6747.
Covello, P.S., and Reed, D.W. (1996) Functional Expression of the Extraplastidial Arabidopsis thaliana Oleate Desaturase Gene (FAD2) in Saccharomyces cerevisiae, Plant Physiol. 111, 223–226.
Kajiwara, S., Shirai, A., Fujii, T., Toguri, T., Nakamura, K., and Ohtaguchi, K. (1996) Polyunsaturated Fatty Acid Biosynthesis in Saccharamyces cerevisiae: Expression of Ethanol Tolerance and the FAD2 Gene from Arabidopsis thaliana, Appl. Env. Microbiol. 62, 4309–4313.
Knutzon, D.S., Lardizabal, K.D., Nelsen, J.S., Bleibaum, J.L., Davies, H.M., and Metz, J.G. (1995) Cloning of a Coconut Endosperm cDNA Encoding a 1-Acyl-sn-glycerol-3-phosphate Acyltransferase That Accepts Medium-Chain-Length Substrates, Plant Physiol. 109, 999–1006.
Hoveland, P., Flick, J., Johnston, M., and Sclafani, R.A. (1989) Galactose as a Gratuitous Inducer of GAL Gene Expression in Yeasts Growing on Glucose, Gene 83, 57–64.
Sterns, T., Ma, H., and Botstein, D. (1990) Manipulating Yeast Genome Using Plamid Vectors, Meth. Enzymol. 185, 280–297.
Sperling, P., Schmidt, H., and Heinz, E. (1995) A Cytochrome-b5-Containing Fusion Protein Similar to Plant Acyl Lipid Desaturases, Eur. J. Biochem. 232, 798–805.
Stukey, J.E., McDonough, V.M., and Martin, C.E. (1990) The OLE1 Gene of Saccharomyces cerevisiae Encodes the Δ9-Fatty Acid Desaturase and Can Be Functionally Replaced by the Rat Stearoyl-CoA Desaturase Gene, J. Biol. Chem. 265, 20144–20149.
Falcone, D.L., Gibson, S., Lemieux, B., and Somerville, C. (1994) Identification of a Gene That Complements an Arabidopsis Mutant Deficient in Chloroplast ω6 Desaturase Activity, Plant Physiol. 106, 1453–1459.
Schmidt, H., Dresselhaus, T., Buck, F., and Heinz, E. (1994) Purification and PCR-Based cDNA Cloning of a Plastidial n−6 Desaturase, Plant Mol. Biol. 26, 631–642.
van de Loo, F.J., and Somerville, C. (1994) Plastid ω-3 Fatty Acid Desaturase cDNA from Ricinus communis, Plant Physiol. 105, 443–444.
Sakamoto, T., Los, D.A., Higashi, S., Wada, H., Nishida, I., Ohmori, M., and Murata, N. (1994) Cloning of ω3 Desaturase from Cyanobacteria and Its Use in Altering the Degree of Membrane-Lipid Unsaturation, Plant Mol. Biol. 26, 249–263.
Spychalla, J.P., Kinney, A.J., and Browse, J. (1997) Identification of an Animal ω-3 Fatty Acid Desaturase by Heterologous Expression in Arabidopsis, Proc. Natl. Acad. Sci. USA 94, 1142–1147.
Aguilar, P.S., Cronan, J.E., Jr., and de Mendoza, D. (1998) A Bacillus subtilis Gene Induced by Cold Shock Encodes a Membrane Phospholipid Desaturase, J. Bacteriol. 180, 2194–2200.
Watts, J.L., and Browse, J. (1999) Isolation and Characterization of a Δ6-Fatty Acid Desaturase from Caenorhabditis elegans, Arch. Biochem. Biophys. 362, 175–182.
Cho, H.P., Nakamura, M.T., and Clarke, S.D. (1999) Cloning, Expression, and Nutritional Regulation of the Mammalian Δ-6 Desaturase, J. Biol. Chem. 274, 471–477.
Girke, T., Schimdt, H., Zahringer, U., Reski, R., and Heinz, E. (1998) Identification of a Novel Δ6-Acyl-Group Desaturase by Targeted Gene Disruption in Physcomitrella patens, Plant J. 15, 39–48.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Huang, YS., Chaudhary, S., Thurmond, J.M. et al. Cloning of Δ12- and Δ6-desaturases from Mortierella alpina and recombinant production of γ-linolenic acid in Saccharomyces cerevisiae . Lipids 34, 649–659 (1999). https://doi.org/10.1007/s11745-999-0410-8
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-999-0410-8