Abstract
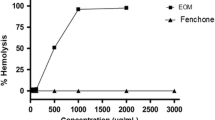
The cytotoxicity of fatty acids from seed oils containing conjugated linolenic acids (CLN) was studied. Fatty acids from pomegranate, tung, and catalpa were cytotoxic to human monocytic leukemia cells at concentrations exceeding 5 μM for pomegranate and tung and 10 μM for catalpa, but fatty acids from pot marigold oil had no effect at concentrations ranging up to 163 μM. The main conjugated fatty acids of pomegranate, tung, catalpa, and pot marigold were cis(c)9, trans(t)11, c13-CLN (71.7%), c9,t11,t13-CLN (70.1%), t9,t11,c13-CLN (31.3%), and t8,t10,c12-CLN (33.4%), respectively. Therefore, the cytotoxicities of fatty acids from pomegranate, tung, and catalpa were supposed to be due to 9,11,13-CLN isomers. To elucidate the cytotoxicity of these CLN, we separated each CLN isomer from the fatty acid mixtures by high-performance liquid chromatography and analyzed its cytotoxicity. The cytotoxicities of c9,t11,c13-CLN, c9,t11,t13-CLN, and t9,t11,c13-CLN were much stronger than that of t8,t10,c12-CLN. Therefore, the higher cytotoxicity of fatty acids from pomegranate, tung, and catalpa than those from pot marigold would be derived from the different activities of 9,11,13-CLN and 8,10,12-CLN. Since there was little difference in the cytotoxicities of c9,t11,c13-CLN, c9,t11,t13-CLN, and t9,t11,c13-CLN, it is suggested that the cis/trans configuration of 9,11,13-CLN isomers had little effect on their cytotoxic effects. The mechanism of the cytotoxicity of the four fatty acids above may involve lipid peroxidation, because the order of toxicity of the fatty acids was consistent with their susceptibility to peroxidation in aqueous phase. This was supported by the decrease in the cytotoxicity of the fatty acids by addition of butylated hydroxytoluene.
Similar content being viewed by others
Abbreviations
- AAPH:
-
2,2′-azobis(2-amidinopropane)dihydrochloride
- BHT:
-
butylated hydroxytoluene
- CLA:
-
conjugated linoleic acid
- CLN:
-
conjugated linolenic acid
- DMEM:
-
Dulbecco's modified Eagle's medium
- GC:
-
gas chromatography
- HPLC:
-
high-performance liquid chromatography
- IsoLN:
-
isomerized linolenic acid
- LA:
-
linoleic acid
- LN:
-
α-linolenic acid
- PUFA:
-
polyunsaturated fatty acid
- WST:
-
water-soluble tetrazolium
References
Ip, C., Scimeca, J.A., and Thompson, H.J. (1994) Conjugated Linoleic Acid, Cancer 74, 1050–1054.
Belury, M.A. (1995) Conjugated Dienoic Linoleate: A Polyunsaturated Fatty Acid with Unique Chemoprotective Properties, Nutr. Rev. 53, 83–89.
Haumann, B.F. (1996) Conjugated Linoleic Acid Offers Research Promise, Inform 7, 152–159.
Park, Y., Albright, K.J., Liu, W., Storkson, J.M., Cook, M.E., and Pariza, M.W. (1997) Effect of Conjugated Linoleic Acid on Body Composition in Mice, Lipids 32, 853–858.
Li, Y., and Watkins, B.A. (1998) Conjugated Linoleic Acids Alter Bone Fatty Acid Composition and Reduce ex vivo Prostaglandin E2 Biosynthesis in Rats Fed n-6 or n-3 Fatty Acids, Lipids 33, 417–425.
Sugano, M., Tsujita, A., Yamasaki, M., Noguchi, M., and Yamada, K. (1998) Conjugated Linoleic Acid Modulates Tissue Levels of Chemical Mediators and Immunoglobulins in Rats, Lipids 33, 521–527.
Park, Y., Storkson, J.M., Albright, K.J., Liu, W., and Pariza, M.W. (1999) Evidence That the trans-10, cis-12 Isomer of Conjugated Linoleic Acid Induces Body Composition Changes in Mice, Lipids 34, 235–241.
Fritsche, J., and Steinhart, H. (1998) Analysis, Occurrence, and Physiological Properties of Trans Fatty Acids (TFA) with Particular Emphasis on Conjugated Linoleic Acid Isomers (CLA)—A Review, Fett/Lipid 100, 190–210.
Shultz, T.D., Chew, B.P., Seaman, W.R., and Luedecke, L.O. (1992) Inhibitory Effect of Conjugated Dienoic Derivatives of Linoleic Acid and β-Carotene on the in vitro Growth of Human Cancer Cells, Cancer Lett. 63, 125–133.
Schonberg, S., and Erokan, H.E. (1995) The Inhibitory Effect of Conjugated Dienoic Derivatives (CLA) of Linoleic Acid on the Growth of Human Tumor Cell Lines Is in Part Due to Increased Lipid Peroxidation. Anticancer Res. 15, 1241–1246.
Bagby, M.O., Smith, C.R., and Wolff, I.A. (1966) Stereochemistry of α-Parinaric Acid from Impatiens edgeworthii Seed Oil, Lipids 1, 263–267.
Smith, C.R. (1971) Occurrence of Unusual Fatty Acids in Plants, Prog. Chem. Fats Other Lipids, 11, 137–177.
Badami, R.C., and Patil, K.B. (1981) Structure and Occurrence of Unusual Fatty Acids in Minor Seed Oils, Prog. Lipid Res. 19, 119–153.
Takagi, T., and Itabashi, Y. (1981) Occurrence of Mixtures of Geometrical Isomers of Conjugated Octadecatrienoic Acids in Some Seed Oils: Analysis by Open-Tubular Gas Liquid Chromatography and High Performance Liquid Chromatography, Lipids 16, 546–551.
Cornelius, A.S., Yerram, N.R., Kratz, D.A., and Spector, A.A. (1991) Cytotoxic Effect of cis-Parinaric Acid in Cultured Malignant Cells, Cancer Res. 51, 6025–6030.
Dhar, P., Ghosh, S., and Bhattacharyya, D.K. (1999) Dietary Effects of Conjugated Octadecatrienoic Fatty Acid (9cis, 11trans, 13trans) Levels on Blood Lipids and Nonenzymatic in vitro Lipid Peroxidation in Rats, Lipids 34, 109–114.
Igarashi, M., and Miyazawa, T. (2000) Newly Recognized Effect of Conjugated Trienoic Fatty Acids on Cultured Human Tumor Cells, Cancer Lett. 148, 173–179.
Igarashi, M., and Miyazawa T. (2000) Do Conjugated Eicosapentaenoic Acid and Conjugated Docosahexaenoic Acid Induce Apoptosis via Lipid Peroxidation in Cultured Human Tumor Cells? Biochem. Biophys. Res. Commun. 270, 649–656.
Takagi, T., Mitsuno, Y., and Masumura, M. (1978) Determination of Peroxide Value by the Colorimetric Iodine Method with Protection of Iodide as Cadmium Complex, Lipids 13, 147–151.
AOCS Official Method Cd 7–58 (1998) Official Methods and Recommended Practices of the American Oil Chemists' Society (Firestone, D., ed.) 5th edn., AOCS Press, Champaign.
Ishiyama, M., Shiga, M., Sakamoto, K., Mizoguchi, M., and He, P. (1993) A New Sulfonated Tetrazolium Salt That Produces a Highly Water-Soluble Formazon Dye, Chem. Pharm. Bull. 41, 1118–1122.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Suzuki, R., Noguchi, R., Ota, T. et al. Cytotoxic effect of conjugated trienoic fatty acids on mouse tumor and human monocytic leukemia cells. Lipids 36, 477–482 (2001). https://doi.org/10.1007/s11745-001-0746-0
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-001-0746-0