Abstract
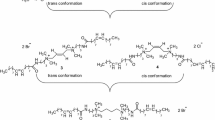
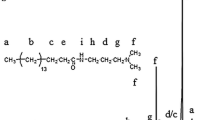
Experimental studies were conducted to investigate thermal and interfacial properties of two in-house synthesized amido-amine-based cationic gemini surfactants namely: dodecanoic acid [3-({4-[(3-dodecanoylamino-propyl)-dimethyl-amino]-butyl}-dimethyl-amino)-propyl]-amide dibromide (12-4-12) and dodecanoic acid [3-({6-[(3-dodecanoylamino-propyl)-dimethyl-amino]-hexyl}-dimethyl-amino)-propyl]-amide dibromide (12-6-12). Thermogravimetric analysis showed the excellent thermal stability of surfactants and no structural degradation was observed at temperatures up to 250 °C. The long-term thermal stability of the surfactants was investigated with the aid of spectroscopic techniques such as nuclear magnetic resonance (NMR (1H and 13C) and Fourier transform infrared (FTIR) spectroscopy. Both surfactants were found to be thermally stable, and no changes in structure were observed after aging for 10 days at 90 °C. The interfacial tension of the surfactants was measured at three different temperatures (30, 60, and 80 °C), and the results showed a decrease in interfacial tension with increasing temperature and increasing spacer length of the surfactants. Rheological measurements were used to assess the interactions between the cationic gemini surfactant and cationic polyacrylamide. The addition of cationic surfactant reduced the viscosity and storage modulus of the polymer at low shear rate and frequency due to surfactant–polymer interactions and charge screening. The investigated surfactant–polymer system has great potential in high-temperature carbonate reservoirs, where conventional anionic surfactants are not recommended due to high adsorption.













Similar content being viewed by others
References
Sheng JJ. Status of surfactant EOR technology. Petroleum. 2015;1(2):97–105. doi:10.1016/j.petlm.2015.07.003.
Al-Amodi AO, Al-Mubaiyedh UA, Sultan AS, Kamal MS, Hussein IA. Novel fluorinated surfactants for enhanced oil recovery in carbonate reservoirs. Can J Chem Eng. 2016;94(3):454–60. doi:10.1002/cjce.22406.
Parker AP, Reynolds PA, Lewis AL, Hughes L. Semi-continuous emulsion co-polymerisation of methylmethacrylate and butylacrylate using zwitterionic surfactants as emulsifiers: evidence of coagulative nucleation above the critical micelle concentration. Colloids Surf A Physicochem Eng Asp. 2005;268(1):162–74.
Szymczyk K, Zdziennicka A, Jańczuk B. Adsorption and wetting properties of cationic, anionic and nonionic surfactants in the glass-aqueous solution of surfactant-air system. Mater Chem Phys. 2015;162:166–76. doi:10.1016/j.matchemphys.2015.05.054.
Prince M. Experimental study on nonionic surfactants for minimizing surface adsorption as an improved oil recovery (IOR) process. Indian J Sci Technol. 2014;7:78.
Shukla D, Tyagi V. Cationic gemini surfactants: a review. J Oleo Sci. 2006;55(8):381–90.
Shah RA, Chat OA, Maswal M, Rather GM, Dar AA. Rheological response of methylcellulose toward alkanediyl-α, ω-bis(dimethylcetylammonium bromide) surfactants with varying spacer length. Carbohydr Polym. 2016;144:159–67. doi:10.1016/j.carbpol.2016.02.052.
Lu T, Lan Y, Liu C, Huang J, Wang Y. Surface properties, aggregation behavior and micellization thermodynamics of a class of gemini surfactants with ethyl ammonium headgroups. J Colloid Interface Sci. 2012;377(1):222–30.
Zana R. Dimeric (gemini) surfactants: effect of the spacer group on the association behavior in aqueous solution. J Colloid Interface Sci. 2002;248(2):203–20. doi:10.1006/jcis.2001.8104.
Ghumare AK, Pawar BV, Bhagwat SS. Synthesis and antibacterial activity of novel amido-amine-based cationic gemini surfactants. J Surfactants Deterg. 2013;16(1):85–93.
Castro Dantas T, Soares AP, Wanderley Neto A, Dantas Neto A, Barros Neto E. Implementing new microemulsion systems in wettability inversion and oil recovery from carbonate reservoirs. Energy Fuels. 2014;28(11):6749–59.
Sheng JJ. Chapter 7 - Surfactant flooding. In: Sheng JJ, editor. Modern chemical enhanced oil recovery. Boston: Gulf Professional Publishing; 2011. p. 239–335.
Hou J, Pan G, Lu X, Wei C, Qiu M. The distribution characteristics of additional extracted oil displaced by surfactant–polymer flooding and its genetic mechanisms. J Pet Sci Eng. 2013;112:322–34. doi:10.1016/j.petrol.2013.11.021.
Sheng JJ, editor. Enhanced oil recovery field case studies. Waltham, MA: Gulf Professional Publishing; 2013. p. 685.
Wever DAZ, Picchioni F, Broekhuis AA. Polymers for enhanced oil recovery: a paradigm for structure–property relationship in aqueous solution. Prog Polym Sci. 2011;36(11):1558–628. doi:10.1016/j.progpolymsci.2011.05.006.
Zhong C, Luo P, Ye Z, Chen H. Characterization and solution properties of a novel water-soluble terpolymer for enhanced oil recovery. Polym Bull. 2009;62(1):79–89. doi:10.1007/s00289-008-1007-6.
Samanta A, Ojha K, Sarkar A, Mandal A. Surfactant and surfactant–polymer flooding for enhanced oil recovery. Adv Pet Explor Dev. 2011;2(1):13–8.
Khan MY, Samanta A, Ojha K, Mandal A. Interaction between aqueous solutions of polymer and surfactant and its effect on physicochemical properties. Asia-Pac J Chem Eng. 2008;3(5):579–85.
Samanta A, Bera A, Ojha K, Mandal A. Effects of alkali, salts, and surfactant on rheological behavior of partially hydrolyzed polyacrylamide solutions. J Chem Eng Data. 2010;55(10):4315–22. doi:10.1021/je100458a.
Jang HY, Zhang K, Chon BH, Choi HJ. Enhanced oil recovery performance and viscosity characteristics of polysaccharide xanthan gum solution. J Ind Eng Chem. 2015;21:741–5. doi:10.1016/j.jiec.2014.04.005.
Ko KM, Chon BH, Jang SB, Jang HY. Surfactant flooding characteristics of dodecyl alkyl sulfate for enhanced oil recovery. J Ind Eng Chem. 2014;20(1):228–33. doi:10.1016/j.jiec.2013.03.043.
Chen H-x, Tang H-m, Gong X-p, Wang J-j, Liu Y-g, Duan M, Zhao F. Effect of partially hydrolyzed polyacrylamide on emulsification stability of wastewater produced from polymer flooding. J Pet Sci Eng. 2015;133:431–9. doi:10.1016/j.petrol.2015.06.031.
Kamal MS, Sultan AS, Al-Mubaiyedh UA, Hussein IA. Review on polymer flooding: rheology, adsorption, stability, and field applications of various polymer systems. Polym Rev. 2015;55(3):491–530. doi:10.1080/15583724.2014.982821.
Zhu D, Zhang J, Han Y, Wang H, Feng Y. Laboratory study on the potential EOR use of HPAM/VES hybrid in high-temperature and high-salinity oil reservoirs. J Chem. 2013;. doi:10.1155/2013/927519.
Kästner U, Zana R. Interactions between quaternary ammonium surfactant oligomers and water-soluble modified guars. J Colloid Interface Sci. 1999;218(2):468–79.
Chu Z, Feng Y. A facile route towards the preparation of ultra-long-chain amidosulfobetaine surfactants. Synlett. 2009;16:2655–8.
Zana R, Benrraou M, Rueff R. Alkanediyl-. alpha., omega.-bis (dimethylalkylammonium bromide) surfactants. 1. Effect of the spacer chain length on the critical micelle concentration and micelle ionization degree. Langmuir. 1991;7(6):1072–5.
Dong Z, Zheng Y, Zhao J. Synthesis, physico-chemical properties and enhanced oil recovery flooding evaluation of novel zwitterionic gemini surfactants. J Surfactants Deterg. 2014;17(6):1213–22.
Zaky MF, Badawi AM, El Sabbah I, Ghani RAA, Hendawy ME. Synthesis, characterization and surface activities of cationic polysaccharide (Aloe) schiff base surfactants. J Surfactants Deterg. 2015;18(3):455–61.
Wu Y, Iglauer S, Shuler P, Tang Y, Goddard W III. Branched alkyl alcohol propoxylated sulfate surfactants for improved oil recovery. Tenside Surfactants Deterg. 2010;47(3):152–61.
Aoudia M, Al-Maamari RS, Nabipour M, Al-Bemani AS, Ayatollahi S. Laboratory study of alkyl ether sulfonates for improved oil recovery in high-salinity carbonate reservoirs: a case study. Energy Fuels. 2010;24(6):3655–60.
Yang HT, Britton C, Liyanage PJ, Solairaj S, Kim DH, Nguyen QP, Weerasooriya U, Pope GA. Low-cost, high-performance chemicals for enhanced oil recovery. In: SPE improved oil recovery symposium. Society of Petroleum Engineers; 2010.
Gao B, Sharma MM. A new family of anionic surfactants for enhanced-oil-recovery applications. SPE J. 2012;18:8–10.
Chen H, Han L, Luo P, Ye Z. The ultralow interfacial tensions between crude oils and gemini surfactant solutions. J Colloid Interface Sci. 2005;285(2):872–4. doi:10.1016/j.jcis.2004.11.066.
Kamal MS. A review of gemini surfactants: potential application in enhanced oil recovery. J Surfactants Deterg. 2016;19(2):1–14. doi:10.1007/s11743-015-1776-5.
Ye Z, Zhang F, Han L, Luo P, Yang J, Chen H. The effect of temperature on the interfacial tension between crude oil and gemini surfactant solution. Colloid Surf A. 2008;322(1–3):138–41. doi:10.1016/j.colsurfa.2008.02.043.
Yao C, Lei G, Hou J, Xu X, Wang D, Steenhuis TS. Enhanced oil recovery using micron-size polyacrylamide elastic microspheres: underlying mechanisms and displacement experiments. Ind Eng Chem Res. 2015;54(43):10925–34.
Wang D, Xia H, Liu Z, Yang Q. Study of the mechanism of polymer solution with visco-elastic behavior increasing microscopic oil displacement efficiency and the forming of steady “Oil thread” flow channels. In: SPE Asia Pacific oil and gas conference and exhibition. Society of Petroleum Engineers; 2001.
Wang D, Cheng J, Xia H, Li Q, Shi J. Viscous-elastic fluids can mobilize oil remaining after water-flood by force parallel to the oil-water interface. In: SPE Asia Pacific improved oil recovery conference. Society of Petroleum Engineers; 2001
Mandal A, Ojha K. Optimum formulation of alkaline-surfactant–polymer systems for enhanced oil recovery. In: SPE Asia Pacific oil and gas conference and exhibition. Society of Petroleum Engineers; 2008.
Bu HT, Yang ZZ, Huang L. Effect of thermal history on rheological properties of partially hydrolyzed polyacrylamide/anionic surfactant SDS complex systems. Chin Chem Lett. 2002;13(5):456–9.
Kamal MS, Hussien IA, Sultan AS, Han M. Rheological study on ATBS-AM copolymer–surfactant system in high-temperature and high-salinity environment. J Chem. 2013;2013:1–9. doi:10.1155/2013/801570.
Urbissinova TS, Trivedi JJ, Kuru E. Effect of elasticity during viscoelastic polymer flooding: a possible mechanism of increasing the sweep efficiency. In: SPE Western Regional Meeting, 27–29 May. Socoiety of Petroleum Engineers, Anaheim; 2010. doi:10.2118/133471-MS.
Zhang Z, Li J, Zhou J. Microscopic roles of “viscoelasticity” in HPMA polymer flooding for EOR. Transp Porous Med. 2011;86(1):199–214. doi:10.1007/s11242-010-9616-6.
Zakharova LY, Gabdrakhmanov DR, Ibragimova AR, Vasilieva EA, Nizameev IR, Kadirov MK, Ermakova EA, Gogoleva NE, Faizullin DA, Pokrovsky AG. Structural, biocomplexation and gene delivery properties of hydroxyethylated gemini surfactants with varied spacer length. Colloids Surf B Biointerfaces. 2016;140:269–77.
Acknowledgements
This work was supported by the Center for Integrative Petroleum Research, King Fahd University of Petroleum and Minerals, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Shakil Hussain, S.M., Kamal, M.S. & Sultan, A.S. Amido-Amine-Based Cationic Gemini Surfactants: Thermal and Interfacial Properties and Interactions with Cationic Polyacrylamide. J Surfact Deterg 20, 47–55 (2017). https://doi.org/10.1007/s11743-016-1896-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-016-1896-6