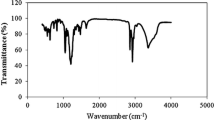
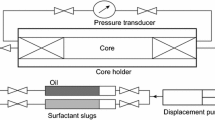
Abstract
In this work, a novel series of zwitterionic gemini surfactants with different hydrophobic tails were synthesized and characterized. The physico-chemical properties of these products (such as surface tension, oil/water interfacial tension, foaming ability, and the wetting ability of paraffin-coated sandstone) were fully studied. The CMC of the synthesized surfactants ranged from 2.17 × 10−4 mol L−1 to 5.36 × 10−4 mol L−1 and corresponding surface tension (γ CMC) ranged from 26.49 mN m−1 to 29.06 mN m−1, which showed excellent efficiency among the comparison surfactants. All the products can reduce the interfacial tension to a relatively low level of about 0.1–1.0 mN m−1. Additionally, results from applying different hydrocarbons suggested that the synergy will be clearer and oil/water interfacial tension will be lower if the oil components are similar to the surfactants. Contact angle and foaming measurements indicated that the surfactants exhibited good wetting and foaming abilities. The results of oil flooding experiments using an authentic sandstone microscopic model showed that C-12 and CA-12 could effectively improve the displacement efficiency by 21–29 %.








Similar content being viewed by others
References
Bunton CA, Robison LB, Schack J, Stam MF (1971) Catalysis of nucleophilic substitutions by micelles of dicationic detergent. J Org Chem 36:2346–2350
Menger FM, Littau CA (1991) Gemini surfactants: synthesis and properties. J Am Chem Soc 113:1451–1452
Afshar S, Yeung A (2012) Considerations when determining low interfacial tensions. J Colloid Interface Sci 364:276–278
Sorenson GP, Coppage KL, Mahanthappa MK (2011) Unusually stable aqueous lyotropic gyroid phases from gemini dicarboxylate surfactants. J Am Chem Soc 133:14928–14931
Snežana S, Christian W, Miroslav MS, Christel M (2009) Natural surfactant-based topical vehicles for two model drugs: influence of different lipophilic excipients on in vitro/in vivo skin performance. Int J Pharm 381:220–230
Ma CZ, Han L, Jiang Z, Huang ZH, Feng J, Yao Y, Che SN (2011) Growth of mesoporous silica film with vertical channels on substrate using gemini surfactants. Chem Mater 23:3583–3586
Menger FM, Keiper JS (2000) Gemini surfactants. Angew Chem Int Ed 39:1906
Roberts GG (1990) Langmuir-Blodgett films. Plenum Press, New York
Guo YJ, Liu JX, Zhang XM et al (2012) Solution property investigation of combination flooding systems consisting of gemini-non-ionic mixed surfactant and hydrophobically associating polyacrylamide for enhanced oil recovery. Energ Fuels 26:2116–2123
Dong MZ, Ma SZ, Liu Q (2009) Enhanced heavy oil recovery through interfacial instability: a study of chemical flooding for Brintnell heavy oil. Fuel 88:1049–1056
Arne S, Jan MG, Ole JM, Lisa T (1992) Optimization of a surfactant flooding process by core-flood experiments. J Pet Sci Eng 2:117–130
Zana R, Lévy H (1997) Alkanediylz-α, ω-bis(dimethylalkylammonium bromide) surfactants (dimeric surfactants) Part 6. CMC of the ethanediyl-1,2-bis(dimethylalkylammonium bromide) series. Colloids Surf A 127:229–232
Zana R (2002) Dimeric (gemini) surfactants: effect of the spacer group on the association behavior in aqueous solution. J Colloid Interface Sci 248:203–220
Zana R, Talmon Y (1993) Dependence of aggregate morphology on structure of dimeric surfactants. Nature 362:228–230
Jaeger DA, Li B, Clark T Jr (1996) Cleavable double-chain surfactants with one cationic and one anionic head group that form vesicles. Langmuir 12:4314–4316
Chen ZG, Liu GL, Chen MH, Peng YR, Wu MY (2009) Determination of nanograms of proteins based on decreased resonance light scattering of zwitterionic gemini surfactant. Anal Biochem 384:337–342
Singh K, Marangoni DG (2007) Synergistic interactions in the mixed micelles of cationic gemini with zwitterionic surfactants: the pH and spacer effect. J Colloid Interface Sci 315:620–626
Eastoe J, Dalton JS (1998) Dynamic surface tension and micelle structures of dichained phosphatidylcholine structure solutions. Langmuir 14:5719–5724
Peresypkin AV, Menger FM (1999) Zwitterionic geminis. Coacervate formation from a single organic compound. J Org Lett 9:1347–1350
Seredyuk V, Alami E, Nydén M, Holmberg K, Peresypkin AV, Menger FM (2002) Adsorption of zwitterionic gemini surfactants at the air-water and solid-water interfaces. Colloids Surf A 103:245–258
Feng J, Liu XP, Zhang L, Zhao S, Yu JY (2011) Dilational viscoelasticity of the zwitterionic gemini surfactants with polyoxyethylene spacers at the interfaces. J Dispers Sci Technol 32:1537–1546
Huang XJ, Xu ZK, Wan LS, Wang ZG, Wang JL (2005) Surface modification of polyacrylonitrile-based membranes by chemical reactions to generate phospholipid moieties. Langmuir 21:2941–2947
Lankalapalli RS, Eckelkamp JT, Sircar D, Ford DA, Subbaiah PV, Bittman R (2009) Synthesis and antioxidant properties of an unnatural plasmalogen analogue bearing a trans o-vinyl ether linkage. Org Lett 11:2784–2787
Zhang SY, Zou J, Zhang FW, Elsabahy M, Felder SE, Zhu JH, Pochan DJ, Wooley KL (2012) Rapid and versatile construction of diverse and functional nanostructures derived from a polyphosphoester-based biomimetic block copolymer system. J Am Chem Soc 134:18467–18474
Sun YH, Feng YJ, Dong HW, Chen Z, Han LK (2007) Synthesis and aqueous solution properties of homologous gemini surfactant with different head groups. Cent Eur J CheM 5:620–634
Drelich J, Bukka K, Miller JD, Hanson FV (1994) Surface tension of toluene-extracted bitumens from Utah oil sands as determined by Wilhelmy plate and contact angle techniques. Energ Fuels 8:700–704
Rey AD (2000) Modeling the Wilhelmy surface tension method for nematic liquid crystals. Langmuir 16:845–849
Cheng HL, Velankar SS (2009) Controlled jamming of particle-laden interfaces using a spinning drop tensiometer. Langmuir 25:4412–4420
Sarafzadeh P, Hezave AZ, Ravanbakhsh M, Niazi A, Ayatollahi S (2013) Enterobacter cloacae as biosurfactant producing bacterium: differentiating its effects on interfacial tension and wettability alteration mechanisms for oil recovery during MEOR process. Colloids Surf B 105:223–229
Pugh RJ (2007) Foaming in chemical surfactant free aqueous dispersions of anatase (titanium dioxide) particles. Langmuir 23:7972–7980
Yamaguchi M, Kanoh C, Seemork J, Nobukawa S, Yanase K (2012) Effect of foaming method on mechanical properties of aqueous foams prepared from surfactant solution. Ind Eng Chem Res 51:14408–14413
Djuve J, Pugh RJ, Sjoblom J (2001) Foaming and dynamic surface tension of aqueous polymer/surfactants solutions. 1: ethyl(hydroxyethyl) cellulose and sodium dodecyl sulphate. Colloids Surf A 186:189–202
Milne AJB, Amirfazli A (2009) Drop shedding by shear flow for hydrophilic to superhydrophobic surfaces. Langmuir 25:14155–14164
Kashaninejad N, Chan WK, Nguyen N (2012) Eccentricity effect of micropatterned surface on contact angle. Langmuir 28:4793–4799
Negm NA, El-Hashash MA, Mohamed DE et al (2013) Gemini cationic surfactants: synthesis and influence of chemical structure on the surface activity. J Surfact Deterg 16:733–738
Chen M, Luo L, Hu X, Yang J (2013) Synthesis and surface tension study of the spacer chain length effect on the adsorption and micellization properties of a new kind of carboxylate gemini surfactant. J Surfact Deterg 16:327–332
Wu X, Zhao L, Wang X, Wang J, Zhang Y (2013) Synthesis and applications of tri-quaternary ammonium salt gemini surfactant. J Dispers Sci Technol 34:106–110
Yang J, Xie J, Chen G et al (2009) Surface, interfacial and aggregation properties of sulfonic acid-containing gemini surfactants with different spacer lengths. Langmuir 25:6100–6105
Han F, Wang P, Song J et al (2012) A surface rheological study of a glucosamide-based trisiloxane gemini surfactant at the air/water interface. J Dispers Sci Technol 33:1708–1714
Negm NA, Zaki MF, Salem MA (2010) Antibacterial and antifungal activities-surface active properties relation of novel di Schiff base cationic gemini amphiphiles bearing homogeneous hydrophobes. J Dispers Sci Technol 31:1390–1395
Acknowledgments
We are thankful for financial support from the National Natural Science Foundation of China (No. 21171139), Visiting Scholar Foundation of Northwest University and the National Science and Technology Pillar Program of China (No.2007BAB17B02).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Dong, Z., Zheng, Y. & Zhao, J. Synthesis, Physico-Chemical Properties and Enhanced Oil Recovery Flooding Evaluation of Novel Zwitterionic Gemini Surfactants. J Surfact Deterg 17, 1213–1222 (2014). https://doi.org/10.1007/s11743-014-1616-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-014-1616-z