Abstract
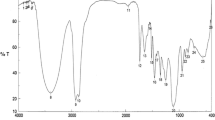
Four nonionic surfactants were prepared from the reaction of propylene oxide with oleic acid, linoleic acid, and the free fatty acid mixture from hydrolysis of jatropha oil. The chemical structures of the prepared surfactants were confirmed using IR and NMR spectroscopy. The surface activities of the prepared surfactants were dependent on the polypropylene oxide chain length and also on the nature of the alkyl chains. The nonionic surfactants were evaluated at different concentrations as corrosion inhibitors against the corrosion of Al 6061 aluminum in 2 M HCl solution. The corrosion inhibition tendencies of the surfactants were completely dependent on the fatty acid ratio in the jatropha oil and also on the polypropylene oxide chain length. The corrosion inhibition efficiencies of the surfactants were correlated to their chemical structure and their surface activities.




Similar content being viewed by others
References
Reznik GO, Vishwanath P, Pynn MA, Sitnik JM, Todd JJ, Wu J, Jiang Y, Keenan BG (2010) Use of sustainable chemistry to produce an acyl amino acid surfactant. Appl Microbiol Biotechnol 86:1387–1397
Li Y, Yang L, Zhu T, Yang J, Ruan X (2013) Biosurfactants as alternatives to chemosynthetic surfactants in controlling bubble behavior in the flotation process. J Surfact Deterg 16:409–419
Negm NA, El Farargy AF, Mohammad IA, Zaki MF, Khowdiary MM (2013) Synthesis and inhibitory activity of Schiff base surfactants derived from tannic acid and their Cobalt (II), Manganese (II) and Iron (III) complexes against bacteria and fungi. J Surfact Deterg 16:767–777
Sonesson AW, Callisen TH, Elofsson UM, Brismar H (2007) Imaging the detergency of single cotton fibers with confocal microscopy: the effect of surfactants and lipases. J Surfact Deterg 10:211–218
Kuntom A, Kifli H (1998) Properties of soaps derived from distilled palm stearin and palm kernel fatty acids. J Surfact Deterg 1:329–334
Berna JL, Moreno A, Bengoechea C (1998) Laundry products in bar form. J Surfact Deterg 1:263–271
Larry N, Britton LN (1998) Surfactants and the environment. J Surfact Deterg 1:109–117
Basu S, Bhattacharyya DK (1998) Utilization of acid oils in making surface-active compounds by lipase-catalyzed hydrolysis and esterification reactions. J Surfact Deterg 1:343–344
O’Lenick AJ Jr (2000) Evaluation of polyoxyethylene glycol esters of castor, high-erucic acid rapeseed, and soybean oils. J Surfact Deterg 3:201–206
Jurado E, Serrano MF, Lechuga M, Ríos F (2012) Environmental impact of ether carboxylic derivative surfactants. J Surfact Deterg 15:1–7
Nakagawa H, Watanabe S, Fujita T, Sakamoto M (1998) Characteristic properties of cutting fluid additives made from the derivatives of some polymeric nonionic surface-active agents. J Surfact Deterg 1:207–211
Zeng X, Lu Z, Liu Y (2013) Synthesis and solution properties of novel sugar-based polysiloxane surfactants. J Surfact Deterg 16:131–137
Abdul-Raheim MA, Abdel-Raouf ME, Maysour NE, El-Kafrawy AF, Mehany AZ, Abdel-Azim AA (2013) Some sugar fatty ester ethoxylates as demulsifiers for petroleum sludge. J Surfact Deterg 16:377–387
Hussein MHM, El-Hady MF, Shehata HAH, Hegazy MA, Hefni HHH (2013) Preparation of some eco-friendly corrosion inhibitors having antibacterial activity from sea food waste. J Surfact Deterg 16:233–242
Palomar ME, Xometl CO, Likhanova NV, Navarrete JB (2011) Imidazolium, pyridinium and dimethyl-ethylbenzyl ammonium derived compounds as mixed corrosion inhibitors in acidic medium. J Surfact Deterg 14:211–220
Sakunthala P, Vivekananthan SS, Gopiraman M, Sulochana N, Vincent AR (2013) Spectroscopic investigations of physicochemical interactions on mild steel in an acidic medium by environmentally friendly green inhibitors. J Surfact Deterg 16:251–263
Negm NA, El-Tabl AS, Aiad IA, Zakareya K, Moustafa AH (2013) Synthesis, characterization, biodegradation and evaluation of the surface active properties of nonionic surfactants derived from jatropha oil. J Surfact Deterg 16:857–863
Sayed GH, Ghuiba FM, Abdou MI, Badr EA, Tawfik SM, Negm NA (2012) Synthesis, surface, thermodynamic properties of some biodegradable Vanillin-modified polyoxyethylene surfactants. J Surfact Deterg 15:735–743
Abdel-Salam FH, El-Said AG (2011) Synthesis and surface active properties of gemini cationic surfactants and interaction with anionic azo dye (AR52). J Surfact Deterg 14:371–379
Han L, Ye Z, Chen H, Luo P (2009) The interfacial tension between cationic gemini surfactant solution and crude oil. J Surfact Deterg 12:185–190
Negm NA, Tawfik SM (2013) Isoxazolium cationic Schiff base surfactants: surface and antimicrobial properties. Chimica Oggi/Chemistry Today 30:5–8
Negm NA, Aiad IA (2007) Synthesis and characterization of multifunctional surfactants in oil-field protection applications. J Surfact Deterg 10:87–92
Negm NA, Kandile NG, Mohamad MA (2011) Synthesis, characterization and surface activity of new eco-friendly Schiff bases Vanillin derived cationic surfactants. J Surfact Deterg 14:325–331
Negm NA, Zaki MF (2008) Corrosion inhibition efficiency of nonionic Schiff base amphiphiles of p-aminobenzoic acid for aluminum in 4 N HCL. Colloids Surf A 322:97–102
Negm NA, El-Hashash MA, Mohamed DE, Marquis JM, Khowdiary MM (2013) Gemini cationic surfactants: synthesis and influence of chemical structure on the surface activity. J Surfact Deterg 16:733–738
Zana R (2002) Dimeric (gemini) surfactants: effect of the spacer group on the association behavior in aqueous solution. J Colloids Interface Sci 248:203–220
Sakunthala P, Vivekananthan SS, Gopiraman M, Sulochana N, Vincent AR (2013) Spectroscopic investigations of physicochemical interactions on mild steel in an acidic medium by environmentally friendly green inhibitors. J Surfact Deterg 16:251–263
Atta AM, Elsockary MA, Kandil OF, Shaker NO (2008) Nonionic surfactants from recycled poly(ethylene terephthalate) as corrosion inhibitors of steel in 1 M HCl. J Disper Sci Technol 29:27–39
Shalaby MN, Osman MM (2002) Application of some commercial nonionic surfactants in the field of corrosion inhibition. Mater Corros 53:827–832
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Negm, N.A., El-Farargy, A.F.M., Abdel Halim, E.A. et al. Novel Biobased Nonionic Surfactants: Synthesis, Surface Activity and Corrosion Inhibition Efficiency Against Aluminum Alloy Dissolution in Acidic Media. J Surfact Deterg 17, 1203–1211 (2014). https://doi.org/10.1007/s11743-014-1599-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-014-1599-9