Abstract
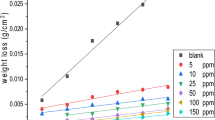
Novel fatty alcohol ethoxylate surfactants (NFAES) C1:(C12–C14–EO30) and C2:(C16–C18–EO30) were synthesized and characterized, and the effect of an increase of the hydrocarbon chain length at the same numbers of ethoxylated groups of these surfactants on the corrosion of mild steel (MS) in 1 M HCl was studied by different methods. These have revealed that the inhibition efficiency (IE%) increases with increasing the dose and the hydrocarbon chain length at the same number of ethoxylated groups in the surfactants (NFAES). The surfactants (NFAES) have a perfect performance as corrosion inhibitors. The results revealed that C2 > C1 in the IE% at the same studied dose of each surfactant, The IE% reached 85% at the very low dose of 30 ppm. Potentiodynamic measurements (PP) revealed that the NFAES surfactants are working as mixed type of inhibitors for both cathodic (reduction) and anodic (oxidation) reactions. The critical micelle dose for C1 is 350 ppm while for C2 it is 30 ppm. IE% acquired from PP, electrochemical impedance spectroscopy, and electrochemical frequency modulation are approximatly the same. The surface morphology was analyzed and is discussed.













Similar content being viewed by others
References
Zhang R, Somasundaran P (2006) Advances in adsorption of surfactants and their mixtures at solid/solution interfaces. Adv Colloid Interface Sci 123:213–229. https://doi.org/10.1016/j.cis.2006.07.004
Zhu Y, Free ML (2015) The effects of surfactant dose, adsorption, aggregation, and solution conditions on steel corrosion inhibition and associated modeling in aqueous media. Corros Sci 102:233–250. https://doi.org/10.1016/j.corsci.2015.10.012
Zhu Y, Free ML (2015) Experimental investigation and modeling of the performance of pure and mixed surfactant inhibitors: partitioning and distribution in water-oil environments. J Electrochem Soc 162:702–717. https://doi.org/10.1149/2.0291514jes
Zhu Y, Free ML, Cho J (2016) Integrated evaluation of mixed surfactants’ distribution in water oil- steel pipe environments and associated corrosion inhibition efficiency. Corros Sci 110:213–227. https://doi.org/10.1016/j.corsci.2016.04.043
Sliem MH, Afifi M, Radwan AB, Fayyad EM, Shibl MF, Heakal FET, Abdullah AM (2019) AEO7 surfactant as an eco-friendly corrosion inhibitor for carbon steel in HCl solution. Nat Sci Rep 9:1–16. https://doi.org/10.1038/s41598-018-37254-7
Abd-Elaal AA, Elbasiony NM, Shaban SM, Zaki EG (2018) Studying the corrosion inhibition of some prepared nonionic surfactants based on 3-(4-hydroxyphenyl) propanoic acid and estimating the influence of silver nanoparticles on the surface parameters. J Mol Liq 249:304–317. https://doi.org/10.1016/j.molliq.2017.11.052
Kibbey TCG, Chen L (2008) Phase volume effects in the sub- and super-CMC partitioning of nonionic surfactant mixtures between water and immiscible organic liquids. Colloids Surf A 326:73–82. https://doi.org/10.1016/j.colsurfa.2008.05.018
Stoyanov SD, Paunov VN, Rehage H, Kuhn H (2004) A new class of interfacial tension isotherms for nonionic surfactants based on local self-consistent mean field theory; classical isotherms revisite. Phys Chem Chem Phys 6:596–603. https://doi.org/10.1039/b314100d
Wang W, Free ML (2004) Prediction and measurement of corrosion inhibition of mild steel using nonionic surfactants in chloride media. Corros Sci 46:2601–2611. https://doi.org/10.1016/s0010-938x(03)00152-5
Abd El-Ghaffar MA, Sherif MH, El-Habab AT (2017) Synthesis, characterization, and evaluation of ethoxylated lauryl-myrisityl alcohol nonionic surfactants as wetting agents, anti-foamers, and minimum film forming temperature reducers in emulsion polymer lattices. J Surfactants Deterg 20:117–128. https://doi.org/10.1007/s11743-016-1898-4
Moreira L, Firoozabadi A (2010) Molecular thermodynamic modeling of specific ion effects on micellization of ionic surfactants. Langmuir 26:15177–15191. https://doi.org/10.1021/la102536y
Valkovska DS, Shearman GC, Bain CD, Darton RC, Eastoe J (2004) Adsorption of ionic surfactants at an expanding air-water interface. Langmuir 20:4436–4445. https://doi.org/10.1021/la035739b
Fuchs-Godec R, Pavlovic MG (2012) Synergistic effect between non-ionic surfactant and halide ions in the forms of inorganic or organic salts for the corrosion inhibition of stainlesssteel X4Cr13 in sulphuric acid. Corros Sci 58:192–201. https://doi.org/10.1016/j.corsci.2012.01.027
Hegazy AY, El-Shafaie M, Berry KM (2016) Novel cationic surfactants for corrosion inhibition of carbon steel pipelines in oil and gas wells applications. J Mol Liq 214:347–356. https://doi.org/10.1016/j.molliq.2015.11.047
Sonu, Tiwari AK, Saha SK (2013) Study on mixed micelles of cationic gemini surfactants having hydroxyl groups in the spacers with conventional cationic surfactants: effects of spacer group and hydrocarbon tail length. Ind Eng Chem Res 52:5895–5905. https://doi.org/10.1021/ie303616j
Shalaby MN, Osman MM, El Feky AA (1999) Effect of some organic surfactants on corrosion inhibition of steel in sea water. Anti-corros Methods Mater 46:254–265. https://doi.org/10.1108/00035599910278660
Rajendran S, Mariajoany R, Apparao BV, Palaniswamy N (2000) Synergistic effect of calcium gluconate and Zn2+ on the inhibition of corrosion of mild steel in neutral aqueous environment. Trans SAEST 35:3
Khan Z (1999) Kinetics and mechanism of the reaction between dimethylformamide and chromium (VI). Int J Chem Kinet 31:409–415. https://doi.org/10.1002/(SICI)1097-4601(1999)31:6%3c409::AID-KIN2%3e3.0.CO;2-8
Shaban SM, El-Sherif RM, Fahim MA (2018) Studying the surface behavior of some prepared free hydroxyl cationic amphipathic compounds in aqueous solution and their biological activity. J Mol Liq 252:40–51. https://doi.org/10.1016/j.molliq.2017.12.105
Sk MH et al (2017) Local supersaturation and the growth of protective scales during CO2 corrosion of steel: effect of pH and solution flow. Corros Sci 126:26–36. https://doi.org/10.1016/j.corsci.2017.05.026
Zhu Y, Free ML, Woollam R, Durnie W (2017) A review of surfactants as corrosion inhibitors and associated modeling. Prog Mater Sci 90:159–223. https://doi.org/10.1016/j.pmatsci.2017.07.006
Kumar R, Yadav OS, Singh G (2017) Electrochemical and surface characterization of a new eco-friendly corrosion inhibitor for mild steel in acidic media: a cumulative study. J Mol Liq 237:413–427. https://doi.org/10.1016/j.molliq.2017.04.103
Nnaji NJN et al (2017) Morpholine and piperazine based carboxamide derivatives as corrosion inhibitors of mild steel in HCl medium. J Mol Liq 230:652–661. https://doi.org/10.1016/j.molliq.2017.01.075
Han T, Guo J, Zhao Q, Wu Y, Zhang Y (2020) Enhanced corrosion inhibition of LCS by pyridyl gemini surfactants with different alkyl chains. Mater Chem Phys 240:122156. https://doi.org/10.1016/j.matchemphys.2019.122156
Fouda AS, Al-Zehry HH, Elsayed M (2018) Synergistic effect of potassium iodide with cassia italica extract on the corrosion inhibition of carbon steel used in cooling water systems in 0.5 M H2SO4. J Bio- Tribo-Corros 4:23. https://doi.org/10.1007/s40735-018-0138-z
Heakal FE, Elkholy AE (2017) Gemini surfactants as corrosion inhibitors for carbon steel. J Mol Liq 230:395–407. https://doi.org/10.1016/j.molliq.2017.01.047
Wu J, Cai G, Liu J, Ge H, Wang J (2014) Eco-friendly surface modification on polyester fabrics by esterase treatment. Appl Surf Sci 295:150–157. https://doi.org/10.1016/j.apsusc.2014.01.019
Kousar K, Ljungdahl T, Wetzel A, Dowhyj M, Oskarsson H, Walton AS, Lindsay R (2020) An exemplar imidazoline surfactant for corrosion inhibitor studies: synthesis, characterization, and physicochemical properties. J Surfactants Deterg 23:225–234. https://doi.org/10.1002/jsde.12363
Deyab MA (2018) Efficiency of cationic surfactant as microbial corrosion inhibitor for carbon steel in oilfield saline water. J Mol Liq 255:550–555. https://doi.org/10.1016/j.molliq.2018.02.019
Reichenbacher M, Popp J (2012) Vibrational spectroscopy. Challenges in molecular structure determination. Springer, Berlin
Cowell MA, Kibbey TCG, Zimmerman JB, Hayes KF (2000) Partitioning of ethoxylated nonionic surfactants in water/NAPL systems: effects of surfactants and NAPL properties. Environ Sci Technol 34:1583–1588. https://doi.org/10.1021/es9908826
Bianchetti CLDGO, Seddon KR (2015) Bleaching systems in domestic laundry detergents: a review. RSC Adv 5:65365–65384. https://doi.org/10.1039/C5RA05328E
Fuchs-Godec R (2009) Effects of surfactants and their mixtures on inhibition of the corrosion process of ferritic stainless steel. Electrochim Acta 54:2171–2179. https://doi.org/10.1016/j.electacta.2008.10.014
Abdallah M (2003) Ethoxylated fatty alcohols as corrosion inhibitors for dissolution of zinc in hydrochloric acid. Corros Sci 45:2705–2716. https://doi.org/10.1016/s0010-938x(03)00107-0
Mobin M et al (2017) Bio-/environment-friendly cationic gemini surfactant as novel corrosion inhibitor for mild steel in 1 M HCl solution. J Surfactants Deterg 20:57–74. https://doi.org/10.1007/s11743-016-1904-X
Samy AAA-E, Shaban M, Salah MT (2016) Gravimetric and electrochemical evaluation of three nonionic dithiol surfactants as corrosion inhibitors for mild steel in 1 M HCl solution. J Mol Liq 216:392–400. https://doi.org/10.1016/j.molliq.2016.01.048
Ghoulam B, Moatadid N, Graciaa A, Lachaise J (2002) Effects of oxyethylene chain length and temperature on partitioning of homologous polyoxyethylene nonionic surfactants between water and isooctane. Langmuir 18:4367–4371. https://doi.org/10.1021/la0117707
Ghoulam MB, Moatadid N, Graciaa A, Lachaise J (2004) Quantitative effect of nonionic surfactant partitioning on the hydrophile-lipophile balance temperature. Langmuir 20:2584–2589. https://doi.org/10.1021/la030306u
Kokalj A, Peljhan S, Finsgar M, Milosev I (2010) What determines the inhibition effectiveness of ATA, BTAH, and BTAOH corrosion inhibitors on copper. J Am Chem Soc 132:16657–16668. https://doi.org/10.1021/ja107704y
Gece G (2011) Drugs: a review of promising novel corrosion inhibitors. Corros Sci 53:3873–3898. https://doi.org/10.1016/j.corsci.2011.08.006
Xu Q, Wang L, Xing F (2011) Synthesis and properties of dissymmetric gemini surfactants. J Surfactants Deterg 14:85–90. https://doi.org/10.1007/s11743-010-1207-6
Zhu Y, Free ML, Yi G (2015) Electrochemical measurement, modeling, and prediction of corrosion inhibition efficiency of ternary mixtures of homologous surfactants in salt solution. Corros Sci 98:417–429. https://doi.org/10.1016/j.corsci.2015.05.050
Zhang S, Tao Z, Liao S, Wu F (2010) Substitutional adsorption isotherms and corrosion inhibitive properties of some oxadiazol-triazole derivative in acidic solution. Corros Sci 52:3126–3132. https://doi.org/10.1016/j.corsci.2010.05.035
Naderi AHE, Jafari M, Ehteshamzadeh MG, Hosseini G (2009) Effect of carbonsteel microstructures and molecular structure of two new Schiff base compounds on inhibition performance in 1 M HCl solution by EIS. Mater Chem Phys 115:852–858. https://doi.org/10.1016/j.matchemphys.2009.03.002
Shukla SK, Quraishi MA, Prakash R (2008) A self-doped conducting polymer “polyanthranilic acid”: an efficient corrosion inhibitor for mild steel in acidic solution. Corros Sci 50:2867–2872. https://doi.org/10.1016/j.corsci.2008.07.025
Khaled KF, Babić-Samardžija K, Hackerman N (2006) Cobalt (III) complexes of macrocyclic-bidentate type as a new group of corrosion inhibitors for iron in perchloric acid. Corros Sci 48:3014–3034. https://doi.org/10.1016/j.corsci.2005.11.011
Arthur JRHT, Phan L, Jessop PG, Hodson PV (2012) Effects-driven chemical design: the acute toxicity of CO2-triggered switchable surfactants to rainbow trout can be predicted from octanol-water partition coefficients. Green Chem 14:357–362. https://doi.org/10.1039/C1GC15620A
Macdonald JR (1987) Impedance spectroscopy and its use in analyzing the steady-state AC response of solid and liquid electrolytes. J Electroanal Chem Interfacial Electrochem 223:25–50. https://doi.org/10.1016/0022-0728(87)85249-x
Shaban MM, Eid AM, Farag RK, Negm NA, Fadda AA, Migahed MA (2020) Novel trimeric cationic pyrdinium surfactants as bi-functional corrosion inhibitors and antiscalants for API 5L X70 LCS against oilfield formation water. J Mol Liq. https://doi.org/10.1016/j.molliq.2020.112817
Labena A, Hegazy MA, Sami RM, Hozzein WN (2020) Multiple applications of a novel cationic gemini surfactant: anti-microbial, anti-biofilm, biocide, salinity corrosion inhibitor, and biofilm dispersion (Part II). Molecules 25:1348. https://doi.org/10.3390/molecules25061348
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fouda, A.S., El-Maksoud, S.A.A., El-Habab, A.T. et al. Synthesis and Characterization of Novel Fatty Alcohol Ethoxylate Surfactants for Corrosion Inhibition of Mild Steel. J Bio Tribo Corros 7, 18 (2021). https://doi.org/10.1007/s40735-020-00448-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00448-6