Abstract
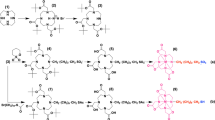
The active site of Candida rugosa lipase (CRL) is mainly hydrophilic on its external face and hydrophobic on the internal side, and calix[n]arene-based surfactants form complexes with protein residues or with strong hydrogen bonds to open up the lid. Therefore, the activity of lipase persists for a long time. In this work, a series of cyclic and acyclic anionic surfactants (sodium dodecyl sulfate (SDS), p-sulfonatocalix[4]arene, and p-sulfonatocalix[8]arene) and zwitterionic surfactants (l-proline and l-proline derivative of calix[4]arene) were used to examine the relationship between the surfactants’ molecular structures and their effects on the hydrolytic activity of CRL. We explored the effects of different surfactant concentrations, ring effects, and mixing times on CRL activity and several kinetic parameters. The results demonstrated that cyclic compounds were more effective than linear structures for increasing CRL activity and the highest enzyme activity was obtained by the addition of the calix[4]-l-proline derivative. This zwitterionic compound (calix[4]-l-proline derivative) maintains the active center of enzyme and conformation by enabling electrostatic interactions and hydrogen bonding with both the acidic and basic amino acid groups in the structure of the enzyme. The results indicated that, compared with the other surfactants, activating CRL with calix[4]-l-proline resulted in hyperactivation at all concentrations (a relative increase of 230%).







Similar content being viewed by others
References
Bornscheuer UT (2003) Immobilizing enzymes: how to create more suitable biocatalysts. Angew Chem Int Ed 42:3336–3337
Dhake KP, Tambade PJ, Qureshi ZS, Singhal RS, Bhanage BM (2011) Hpmc-Pva film immobilized rhizopus oryzae lipase as a biocatalyst for transesterification reaction. ACS Catal 1:316–322
Yildiz H, Ozyilmaz E, Bhatti AA, Yilmaz M (2017) Enantioselective resolution of racemic flurbiprofen methyl ester by lipase encapsulated mercapto calix[4]arenes capped Fe3O4 nanoparticles. Bioprocess Biosyst Eng 40:1189–1196
Zhang DH, Zhang YF, Zhi GY, Xie YL (2011) Effect of hydrophobic/hydrophilic characteristics of magnetic microspheres on the immobilization of BSA. Colloids Surf B Biointerfaces 82:302–306
Zhang W, Tang Y, Liu J, Jiang L, Huang W, Huo FW, Tian D (2015) Colorimetric assay for heterogeneous-catalyzed lipase activity: enzyme-regulated gold nanoparticle aggregation. J Agric Food Chem 63:39–42
Mosmuller EWJ, Franssen MCR, Engbersen JFJ (1993) Lipase activity in vesicular systems: characterization of Candida cylindracea lipase and its activity in polymerizable diaikylammonium surfactant vesicles. Biotechnol Bioeng 42:196–204
Rubingh DN (1996) The influence of surfactants on enzyme activity. Curr Opin Colloid Interface Sci 1:598–603
Gabriele F, Spreti N, Del Giacco T, Germani R, Tiecco M (2018) Effect of surfactant structure on the superactivity of Candida rugosa lipase. Langmuir 34:11510–11517
Lotti M, Tramontano A, Longhi S, Fusetti F, Brocca S, Pizzi E, Alberghina L (1994) Variability within the Candida rugosa lipase family. Protein Eng 7:531–535
Hernández JH, del Pozo JT, Canelo PM, Puebla AR, Tejo SP, Marcos JS (2004) Lung cancer incidence in the Province of Avila, Spain in 2002 and decade-long trends. Arch Bronconeumol 40:304–310
Mathesh M, Luan B, Akanbi TO, Weber JK, Liu J, Barrow CJ, Zhou R, Yang W (2016) Opening lids: modulation of lipase immobilization by graphene oxides. ACS Catal 6:4760–4768
Yilmaz M, Sayin S (2016) Calixarenes in organo and biomimetic catalysis. In: Neri P, Sessler J, Wang MX (eds) Calixarenes and beyond. Springer, Cham
Shinkai S, Mori S, Tsubaki T, Sone T, Mababe O (1984) New water-soluble host molecules derived from calix[6]arene. Tetrahedron Lett 25:5315–5318
Khokhar TS, Memon S, Memon AA, Bhatti AA, Bhatti AA (2020) Improved solubility of morin using p-sulphonatocalix[4]arene as encapsulating agent: hplc analysis and their molecular modelling. Polycy Arom Comp (in press)
Shinkai S, Araki K, Tsubaki T, Arimura R, Manabe O (1987) New syntheses of calixarene-p-sulfonates and p-nitrocalixarenes. J Chem Soc Perkin Trans 1:2297–2299
Arduini A, Pochini A, Reverberi S, Ungaro R (1984) p-t-Butyl-calix[4]arene tetracarboxylic acid. a water soluble calixarene in a cone structure. J Chem Soc Chem Commun 981–982
Nagasaki T, Sisido K, Arimura T, Shinkai S (1992) Novel conformational isomerism of water-soluble calix[4]arenes. Tetrahedron 48:797–804
Shimizu S, Kito K, Sasaki Y, Hirai C (1997) Water-soluble calixarenes as new inverse phase-transfer catalysts. Nucleophilic substitution of alkyl and arylalkyl halides in aqueous media. Chem Commun 1629–1630
Almi A, Arduini A, Casnati A, Pochini A, Ungaro R (1989) Chloromethylation of calixarenes and synthesis of new water-soluble macrocyclic hosts. Tetrahedron 45:2177–2182
Arimura T, Nagasaki T, Shinkai S, Matsuda T (1989) Host-guest properties of new water-soluble calixarenes derived from p-(chloromethyl) calixarenes. J Org Chem 54:3766–3768
Marra A, Scherrmann MC, Dondoni A, Casnati A, Minari P, Ungaro R (1995) Sugar calixarenes: preparation of calix[4]arenes substituted at the lower and upper rims with o-glycosyl groups. Angew Chem Int Ed Eng 33:2479–2481
Dondoni A, Marra A, Scherrmann MC, Casnati F, Sansone F, Ungaro R (1997) Synthesis and properties of o-glycosyl calix[4]arenes (calixsugars). Chem Eur J 3:1774–1782
Roy R, Kim JM (1999) Amphiphilic p-tert-butylcalix[4]arene scaffolds containing exposed carbohydrate dendrons. Angew Chem Int Ed 38:369–372
Pérez-Balderas F, Ortega-Muñoz M, Morales-Sanfrutos J, Hernández-Mateo F, Calvo-Flores FG, Calvo-Asín JA, Isac-García J, Santoyo-González F (2003) Multivalent neoglycoconjugates by regiospecific cycloaddition of alkynes and azides using organic-soluble copper catalysts. Org Lett 5:1951–1954
Consoli GML, Cunsolo F, Geraci C, Sgarlata V (2004) Synthesis and lectin binding ability of glycosamino acid−calixarenes exposing glcnac clusters. Org Lett 6:4163–4166
Hamuro Y, Calama MC, Park HS, Hamilton ADA (1997) Calixarene with four peptide loops: an antibody mimic for recognition of protein surfaces. Angew Chem Int Ed Engl 36:2680–2683
Park HS, Lin Q, Hamilton AD (1999) Protein surface recognition by synthetic receptors: a route to novel submicromolar inhibitors for α-chymotrypsin. J Am Chem Soc 121:8–13
Kellermann M, Bauer W, Hirsch A, Schade B, Ludwig K, Bottcher C (2004) The first account of a structurally persistent micelle. Angew Chem Int Ed 43:2959–2962
Lee M, Lee SJ, Jiang LH (2004) Stimuli-responsive supramolecular nanocapsules from amphiphilic calixarene assembly. J Am Chem Soc 126:12724–12725
Ryu EH, Zhao Y (2004) Environmentally responsive molecular baskets: unimolecular mimics of both micelles and reversed micelles. Org Lett 6:3187–3189
Sayin S, Yilmaz M (2016) Synthesis and investigation of catalytic affinities of water-soluble amphiphilic calix[n]arene surfactants in the coupling reaction of some heteroaromatic compounds. Tetrahedron 72:6528–6535
Savelli G, Spreti N, Di Profio P (2000) Enzyme activity and stability control by amphiphilic self-organizing systems in aqueous solutions. Curr Opin Colloid Interface Sci 5:111–117
Biasutti MA, Abuin EB, Silber JJ, Correa NM, Lissi EA (2008) Kinetics of reactions catalyzed by enzymes in solutions of surfactants. Adv Colloid Interface Sci 136:1–24
Otzen D (2011) Protein-surfactant interactions: a tale of many states. Biochim Biophys Acta 1814:562–591
Sintra TE, Ventura SPM, Coutinho JAP (2014) Superactivity induced by micellar systems as the key for boosting the yield of enzymatic reactions. J Mol Catal B Enzym 107:140–151
Holmberg K (2018) Interactions between surfactants and hydrolytic enzymes. Colloids Surf B Biointerfaces 168:169–177
Mitra RN, Dasgupta A, Das D, Roy S, Debnath S, Das PK (2005) Geometric constraints at the surfactant headgroup: effect on lipase activity in cationic reverse micelles. Langmuir 21:12115–12123
Prazeres JN, Cruz JAB, Pastore GM (2006) Characterization of alkaline lipase from Fusarium oxysporum and the effect of different surfactants and detergents on the enzyme activity. Braz J Microbiol 37:505–509
Becker T, Goh CY, Jones F, McIldowie MJ, Mocerino M, Ogden MI (2008) Proline-functionalised calix[4]arene: an anion-triggered hydrogelator. Chem Commun 33:3900–3902
Chiou SH, Wu WT (2004) Immobilization of Candida rugosa lipase on chitosan with activation of the hydroxyl groups. Biomaterials 25:197–204
Sayin M, Akoz E, Yilmaz M (2014) Enhanced catalysis and enantioselective resolution of racemic naproxen methyl ester by lipase encapsulated within iron oxide nanoparticles coated with calix[8]arene valeric acid complexes. Org Biomol Chem 12:6634–6642
Eriksson T, Börjesson J, Tjerneld F (2002) Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzyme Microb Technol 31:353–364
Coleman AW, Jebors S, Cecillon S, Perret P, Garin D, Marti-Battle D, Moulin M (2008) Toxicity and biodistribution of para-sulfonato-calix[4]arene in mice. New J Chem 32:780–782
Guo DS, Liu Y (2014) Supramolecular chemistry of p-sulfonatocalix[n]arenes and its biological applications. Acc Chem Res 47:1925–1934
Perret F, Coleman AW (2011) Biochemistry of anionic calix[n]arenes. Chem Commun 47:7303–7319
Oshima T, Sato M, Shikaze Y, Ohto K, Inoue K, Baba Y (2007) Enzymatic polymerization of o-phenylenediamine with cytochrome c activated by a calixarene derivative in organic media. Biochem Eng J 35:66–70
Akoz E, Akbulut OY, Yilmaz M (2014) Calix[n]arene carboxylic acid derivatives as regulators of enzymatic reactions: enhanced enantioselectivity in lipase-catalyzed hydrolysis of (r/s)-naproxen methyl ester. Appl Biochem Biotechno 172:509–523
Perret F, Lazar AN, Coleman AW (2006) Biochemistry of the para-sulfonato-calix[n]arenes. Chem Commun 2425–2438
Kang Y, Liu K, Zhang X (2014) Supra-amphiphiles: a new bridge between colloidal science and supramolecular chemistry. Langmuir 30:5989–6001
Ozyilmaz E, Bayrakci M, Yilmaz M (2016) Improvement of catalytic activity of candida rugosa lipase in the presence of calix[4]arene bearing iminodicarboxylic/phosphonic acid complexes modified iron oxide nanoparticles. Bioorg Chem 65:1–8
Gerola AP, Costa PFA, Quina FH, Fiedler HD, Nome F (2017) Zwitterionic surfactants in ion binding and catalysis. Curr Opin Colloid Interface Sci 32:39–47
Cui J, Zhao Y, Liu R, Zhong C, Jia S (2016) Surfactant-activated lipase hybrid nanoflowers with enhanced enzymatic performance. Sci Rep 6:27928
Kartal F (2016) Enhanced esterification activity through interfacial activation and crosslinked immobilization mechanism of Rhizopus oryzae lipase in a nonaqueous medium. Biotechnol Progr 32:899–904
Secundo F, Carrea G, Tarabiono C, Gatti-Lafranconi P, Brocca S, Lotti M, Jaeger KE, Puls M, Eggert T (2006) The lid is a structural and functional determinant of lipase activity and selectivity. J Mol Catal B Enzym 39:166–170
Delorme V, Dhouib R, Canaan S, Fotiadu F, Carriere F, Cavalier JF (2011) Effects of surfactants on lipase structure, activity, and inhibition. Pharm Res 28:1831–1842
Belle V, Fournel A, Woudstra M, Ranaldi S, Prieri F, Thome V, Currault J, Verger R, Guigliarelli B, Carrière F (2007) Probing the opening of the pancreatic lipase lid using site-directed spin labeling and EPR spectroscopy. Biochemistry 46:2205–2214
Acknowledgements
The authors thank the Scientific Research Projects Foundation of Selcuk University (SUBAP Grant number 19201030) for financial support of this work produced from a part of F. Eski’s Master's thesis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author reports no conflicts of interest in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
449_2020_2397_MOESM1_ESM.docx
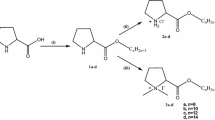
Supplementary file1Synthesis and NMR studies of p-sulfonatocalix[4]arene, p-sulfonatocalix[8]arene and calix [4]arene l-proline derivative. Enzyme activity results at different concentrations of non-cyclic anionic SDS, ring-shaped p-sulfonatocalix[4]arene and p-sulfonatocalix[8]arene, zwitterionic l-proline and calix[4]arene l-proline derivative (DOCX 2246 kb)
Rights and permissions
About this article
Cite this article
Ozyilmaz, E., Eski, F. Effect of cyclic and acyclic surfactants on the activity of Candida rugosa lipase. Bioprocess Biosyst Eng 43, 2085–2093 (2020). https://doi.org/10.1007/s00449-020-02397-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02397-3