Abstract
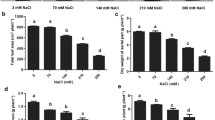
Sugar beet is strongly resistant to salt-alkalinity. Understanding the physiological alkali stress resistance mechanism of sugar beet is important for fully utilizing saline–alkaline soil. Sugar beet seedlings from cultivars KWS0143 (alkali-tolerant) and Beta464 (alkali-sensitive) were treated with five concentrations of mixed alkaline solutions (NaHCO3: Na2CO3, 2:1), namely, 0 (control), 25, 50, 75, and 100 mM (mole concentration was calculated in Na+). A sharper decrease in dry weight per plant (87.1%) and total root length (91.7%) of Beta464 were observed compared to the 61.5% and 85.0% decrease in those of KWS0143 under 100 mM alkali treatment. With increasing alkaline stress, Na+ accumulation hindered K+ and Ca2+ absorption by roots. Free polyamines contents and phosphoenolpyruvate carboxylase (PEPC) activity in roots of both cultivars were all significantly enhanced by 50 and 75 mM alkali treatments. KWS0143 exhibited higher dissociated putrescine (Put), spermine (Spm), as well as spermidine (Spd) levels within the roots compared to Beta464 under alkali conditions. Root free Spd contents of KWS0143 and Beta464 increased by 154.2 and 64.5% treated with 50 mM alkali in comparison with the control. After treated with the dose of 25 mM, root succinic acid (SA) contents of KWS0143 and Beta464 increased by 90.4 and 14.3%, respectively, compared to the plants subjected to the control. Our results imply that polyamines and PEPC contribute to the tolerance of sugar beet to alkali stress. Those results could be useful for enriching the theory of plant stress response.



Similar content being viewed by others
References
Alzahrani SM, Alaraidh IA, Migdadi H, Alghamdi S, Altaf Khan M, Ahmad P (2019) Physiological, biochemical, and antioxidant properties of two genotypes of Vicia faba grown under salinity stress. Pak J Bot. https://doi.org/10.30848/pjb2019-3(3)
An LZ et al (2004) Effect of enhanced UV-B radiation on polyamine content and membrane permeability in cucumber leaves Russian. J Plant Physiol 51:658–662. https://doi.org/10.1023/b:rupp.0000040753.29607.58
An YM, Song LL, Liu YR, Shu YJ, Guo CH (2016) De Novo Transcriptional Analysis of Alfalfa in Response to Saline-Alkaline Stress. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00931
Aziz A, Larher F (1995) Changes in polyamine titers associated with the proline response and osmotic adjustment of rape leaf discs submitted to osmotic stresses. Plant Sci 112:175–186
Azooz MM, Metwally A, Abou-Elhamd MF (2015) Jasmonate-induced tolerance of Hassawi okra seedlings to salinity in brackish water. Acta Physiol Plant 37:1–13
Bai L, Wang C, Zang S, Zhang Y, Hao Q, Wu Y (2016) Remote sensing of soil alkalinity and salinity in the Wuyu’er-Shuangyang river Basin. Northeast China Remote Sens 8:163
Baron K, Stasolla C (2008) The role of polyamines during in vivo and in vitro development. In Vitro Cell Dev Biol Plant 44:384–395
Bouchereau A, Aziz A, Larher F, Martin-Tanguy J (1999) Polyamines and environmental challenges: recent development. Plant Sci 140:103–125
Bueno M, Cordovilla M-P (2019) Polyamines in halophytes. Front Plant Sci. https://doi.org/10.3389/fpls.2019.00439
Capell T, Bassie L, Christou P (2004) Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc Natl Acad Sci USA 101:9909
Chen SS, Xing JJ, Lan HY (2012) Comparative effects of neutral salt and alkaline salt stress on seed germination, early seedling growth and physiological response of a halophyte species Chenopodium glaucum. Afr J Biotechnol 11(40):9572–9581
Chen W et al (2009) Comparative effects of salt and alkali stresses on organic acid accumulation and ionic balance of seabuckthorn (Hippophae rhamnoides L.). Ind Crops Prod 30:351–358. https://doi.org/10.1016/j.indcrop.2009.06.007
Chen YT, Cai-Feng LI, Zhao LY, Peng Y, Wang YY, Teng XY (2010) Screening of Salinity Tolerance And Response Of Seedling To Salt Stress In Sugar Beet (Beta vulgaris L.). Plant Physiol Commun 46:1121–1128
Eshghiizadeh HR, Kafi M, Nezami A, Khoshgoftarmanesh A (2012) Studies on the role of root morphology attribution in salt tolerance of blue-panicgrass (Panicum antidotale Retz) using artificial neural networks (ANN). Res Crops 13:534–544
Fu HJ, Yu HY, Li TX, Wu Y (2019) Effect of cadmium stress on inorganic and organic components in xylem sap of high cadmium accumulating rice line (Oryza sativa L.). Ecotox Environ Safe 168:330–337
Guo J, Li CF, Chen M, Xu Y, Ma FM (2015) Effects of Na2CO3 stress on the growth and antioxidant enzymes of beta vulgaris seedlings. Plant Physiol J 51:840–846
Guo J, Li CF, Liu L, Sang LM, Wang YB (2016) Variation of rhizosphere environmental factors of sugarbeet seedlings under Na2CO3 stress and their correlation. Chin J Appl Ecol 27:904–910
Guo R et al (2017) Ionomic and metabolic responses to neutral salt or alkaline salt stresses in maize (Zea mays L.) seedlings. BMC Plant Biol 17:41. https://doi.org/10.1186/s12870-017-0994-6
Hazman M, Hause B, Eiche E, Riemann M, Nick P (2016) Different forms of osmotic stress evoke qualitatively different responses in rice. J Plant Physiol 202:45–56
Hossain MS, ElSayed AI, Moore M, Dietz KJ (2017) Redox and reactive oxygen species network in acclimation for salinity tolerance in sugar beet. J Exp Bot 68:1283–1298. https://doi.org/10.1093/jxb/erx019
Jesus J, Castro F, Niemelä A, Borges MT, Danko AS (2015) Evaluation of the impact of different soil salinization processes on organic and mineral soils water. Air Soil Pollut 226:1–12
Jiang C-C, Fang Z-Z, Zhou D-R, Pan S-L, Ye X-F (2019) Changes in secondary metabolites, organic acids and soluble sugars during the development of plum fruit cv. ‘Furongli’ (Prunus salicina Lindl). J Sci Food Agric 99:1010–1019. https://doi.org/10.1002/jsfa.9265
Latef AAA, Tran LSP (2016) Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00243
Li H et al (2015) Salt stress response of membrane proteome of sugar beet monosomic addition line M14. J Proteomics 127:18–33. https://doi.org/10.1016/j.jprot.2015.03.025
Li L et al (2018) Exogenously applied spermidine alleviates photosynthetic inhibition under drought stress in maize (Zea mays L.) seedlings associated with changes in endogenous polyamines and phytohormones. Plant Physiol Biochem 129:35–55. https://doi.org/10.1016/j.plaphy.2018.05.017
Li Y, Huang L, Zhang H, Wang M, Liang Z (2017) Assessment of ammonia volatilization losses and ntrogen utilization during the rice growing season in alkaline salt-affected soils. Sustainability 9:132. https://doi.org/10.3390/su9010132
Li Z, Yu J, Peng Y, Huang B (2016) Metabolic pathways regulated by abscisic acid, salicylic acid, and γ-aminobutyric acid in association with improved drought tolerance in creeping bentgrass (Agrostis stolonifera ). Physiol Plant. https://doi.org/10.1111/ppl.12483
Liu D et al (2014) Effect of Zn toxicity on root morphology, ultrastructure, and the ability to accumulate Zn in Moso bamboo (Phyllostachys pubescens). Environ Sci Pollut Res Int 21:13615–13624. https://doi.org/10.1007/s11356-014-3271-3
Liu J, Shi D-C (2010) Photosynthesis, chlorophyll fluorescence, inorganic ion and organic acid accumulations of sunflower in responses to salt and salt-alkaline mixed stress. Photosynthetica 48:127–134. https://doi.org/10.1007/s11099-010-0017-4
Liu JH, Nakajima I, Moriguchi T (2011) Effects of salt and osmotic stresses on free polyamine content and expression of polyamine biosynthetic genes in Vitis vinifera. Biol Plant 55:340–344
Ma B et al (2015) Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chem 172:86–91. https://doi.org/10.1016/j.foodchem.2014.09.032
Ma Y, Wang XP, Zhang SF, Shi DC, Sheng LX (2016) Effects of salt and alkali stress on growth, accumulation of oxalic acid, and activity of oxalic acid-metabolizing enzymes in Kochia sieversiana. Biol Plant 60:774–782. https://doi.org/10.1007/s10535-016-0650-2
Magaña C et al (2011) Direct prediction of bioethanol yield in sugar beet pulp using near infrared spectroscopy. Biores Technol 102:9542
Moschou P, Paschalidis K, Roubelakis-Angelakis K (2008) Plant polyamine catabolism: the state of the art. Plant Signal Behav 3:1061–1066. https://doi.org/10.4161/psb.3.12.7172
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Plant Biology 59:651–681
Parihar P, Singh S, Singh R, Singh VP, Prasad SM (2015) Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Res 22:4056–4075
Paz RC, Rocco RA, Reinoso H, Menéndez AB, Pieckenstain FL, Ruiz OA (2012) Comparative study of alkaline, saline, and mixed saline–alkaline stresses with regard to their effects on growth, nutrient accumulation, and root morphology of lotus tenuis. J Plant Growth Regul 31:448–459. https://doi.org/10.1007/s00344-011-9254-4
Politycka B, Kubiś J (2000) Changes in free polyamine level and di- and polyamine oxidase activity in cucumber roots under allelochemical stress conditions. Acta Physiol Plant 22:11–16
Radi AA, Abdelwahab DA, Hamada AM (2012) Evaluation of some bean lines tolerance to alkaline soil. J Biol Earth Sci 2
Radić S, Prolić M, Pavlica M, Pevalek-Kozlina B (2005) Cytogenetic effects of osmotic stress on the root meristem cells of Centaurea ragusina L. Environ Exp Bot 54:213–218
Shafi M, Guoping Z, Bakht J, AmanKhan M, Islam UE, Khan MD, Raziuddin GZ (2010) Effect of cadmium and salinity stresses on root morphology of wheat. Pak J Bot 42:2747–2754
Smith TA, Barker JH (1985) The DI- and polyamine oxidases of plants. Adv Exp Med Biol 13:319–322
Tang W, Newton RJ (2005) Polyamines reduce salt-induced oxidative damage by increasing the activities of antioxidant enzymes and decreasing lipid peroxidation in Virginia pine. Plant Growth Regul 46:31–43
Vastarelli P, Moschella A, Pacifico D, Mandolino G (2013) Water Stress in Beta vulgaris: Osmotic Adjustment Response and Gene Expression Analysis in ssp. Vulgaris and maritime. Am J Plant Sci 04:11–16
Witzel K et al (2018) Plasma membrane proteome analysis identifies a role of barley membrane steroid binding protein in root architecture response to salinity Plant. Cell Environ 41:1311–1330. https://doi.org/10.1111/pce.13154
Xu Z-M et al (2018) Low root/shoot (R/S) biomass ratio can be an indicator of low cadmium accumulation in the shoot of Chinese flowering cabbage (Brassica campestris L. ssp. chinensis var. utilis Tsen et Lee) cultivars. Environ Sci Pollut Res 25:36328–36340. https://doi.org/10.1007/s11356-018-3566-x
Yang C, Chong J, Li C, Kim C, Shi D, Wang D (2007) Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 294:263–276
Yang C, Shi D, Wang D (2008) Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca (Bge.). Plant Growth Regulat 56:179–190. https://doi.org/10.1007/s10725-008-9299-y
Yang JY, Zheng W, Tian Y, Wu Y, Zhou DW (2011) Effects of various mixed salt-alkaline stresses on growth, photosynthesis, and photosynthetic pigment concentrations of Medicago ruthenica seedlings. Photosynthetica 49:275–284. https://doi.org/10.1007/s11099-011-0037-8
Yang L, Ma C, Wang L, Chen S, Li H (2012) Salt stress induced proteome and transcriptome changes in sugar beet monosomic addition line M14. J Plant Physiol 169:839–850. https://doi.org/10.1016/j.jplph.2012.01.023
Zhang JL, Shi H (2013) Physiological and molecular mechanisms of plant salt tolerance. Photosynth Res 115:1–22
Zhang Y, Fernie AR (2018) On the role of the tricarboxylic acid cycle in plant productivity. J Integr Plant Biol 60:1199–1216. https://doi.org/10.1111/jipb.12690
Zhao J, Shi G, Yuan Q (2008) Polyamines content and physiological and biochemical responses to ladder concentration of nickel stress in Hydrocharis dubia (Bl.) Backer leaves. Biometals 21:665
Zhou Y et al (2019) Application of exogenous glutathione confers salinity stress tolerance in tomato seedlings by modulating ions homeostasis and polyamine metabolism. Sci Hortic 250:45–58. https://doi.org/10.1016/j.scienta.2019.02.026
Zou C, Sang L, Gai Z, Wang Y, Li C (2018) Morphological and physiological responses of sugar beet to alkaline stress. Sugar Tech 20:202–211. https://doi.org/10.1007/s12355-017-0547-1
Zou CL et al (2019) Photosynthetic capacity, osmotic adjustment and antioxidant system in sugar beet (Beta vulgaris L.) in response to alkaline stress. Photosynthetica 57:350–360
Acknowledgements
This work was funded by the National Natural Science Foundation of China (31671622) and Modern Agricultural Industrial Technology System of China (CARS-170201).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. J. Reigosa.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zou, CL., Wang, YB., Wang, B. et al. Effects of alkali stress on dry matter accumulation, root morphology, ion balance, free polyamines, and organic acids of sugar beet. Acta Physiol Plant 43, 13 (2021). https://doi.org/10.1007/s11738-020-03194-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03194-x