Abstract
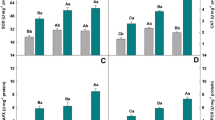
Aluminum (Al) is the third most abundant metal in the Earth’s surface, and Al toxicity promotes several negative effects in plant metabolism. Silicon (Si) is the second most common mineral in soil and is considered a beneficial element for plants, improving their tolerance to biotic and abiotic stresses. The aim of this study is to determine whether Si can reduce the accumulation of Al, explain the possible contribution of Si in mitigating Al toxicity, and indicate the better Si dose–response for cowpea plants. The experiment had a factorial design with two levels of aluminum (0 and 10 mM Al) and three levels of silicon (0, 1.25 and 2.50 mM Si). The utilization of Si in plants exposed to Al toxicity contributed to significant reductions in the Al contents of all tissues, corresponding to reductions of 51, 29 and 41% in roots, stems and leaves, respectively, upon treatment with 2.50 mM Si + 10 mM Al compared to the control treatment (0 mM Si + 10 mM Al). Al toxicity promoted decreases in ΦPSII, qP and ETR, whereas 2.50 mM Si induced increases of 54, 185 and 29%, respectively. Plants exposed to Al had lower values of P N, WUE and P N/C i, whereas Si application at a concentration of 2.50 mM yielded improvements of 53, 32 and 67%, respectively. Al exposure increased SOD, CAT, APX and POX activities, whereas treatment with 2.50 mM Si + 10 mM Al produced significant variations of 72, 97, 48 and 32%, respectively, compared to 0 mM Si + 10 mM Al. Our results proved that Si reduced the Al contents in all tissues. Si also improved the photochemical efficiency of PSII, gas exchange, pigments and antioxidant enzymes, contributing to a reduction in the accumulation of oxidative compounds. These benefits corroborate the multiple roles exercised by Si in metabolism and reveal that Si immobilizes the Al in roots and reduce the accumulation of this metal in other organs, mitigating the damage caused by Al in cowpea plants. In relation to dose–response, plants exposed to 1.25 mM Si without Al presented better results in terms of growth, whereas the toxic effects of plants exposed to Al were mitigated with 2.50 mM Si.




Similar content being viewed by others
Abbreviations
- ΦPSII :
-
Effective quantum yield of PSII photochemistry
- Al:
-
Aluminium
- AsA:
-
Ascorbate
- Ca:
-
Calcium
- CAR:
-
Carotenoids
- Chla :
-
Chlorophyll a
- Chlb :
-
Chlorophyll b
- C i :
-
Intercellular CO2 concentration
- CO2 :
-
Carbon dioxide
- E :
-
Transpiration rate
- EL:
-
Electrolyte leakage
- ETR:
-
Electron transport rate
- ETR/P N :
-
Ratio between the apparent electron transport rate and net photosynthetic rate
- EXC:
-
Relative energy excess at the PSII level
- Fe:
-
Iron
- F m :
-
Maximal fluorescence yield of the dark-adapted state
- F 0 :
-
Minimal fluorescence yield of the dark-adapted state
- F v :
-
Variable fluorescence
- F v/F m :
-
Maximal quantum yield of PSII photochemistry
- g s :
-
Stomatal conductance
- H2O2 :
-
Hydrogen peroxide
- K:
-
Potassium
- MDA:
-
Malondialdehyde
- Mg:
-
Magnesium
- Mn:
-
Manganese
- NPQ:
-
Nonphotochemical quenching
- O2 − :
-
Superoxide
- P N :
-
Net photosynthetic rate
- P N/C i :
-
Instantaneous carboxylation efficiency
- PSII:
-
Photosystem II
- qP :
-
Photochemical quenching
- ROS:
-
Reactive oxygen species
- RUBISCO:
-
ribulose-1,5-bisphosphate carboxylase/oxygenase
- Si:
-
Silicon
- Total Chl:
-
Total chrolophyll
- WUE:
-
Water-use efficiency
- Zn:
-
Zinc
References
Ali S, Bai P, Zeng F, Cai S, Shamsi IH, Qiu B, Wu F, Zhang G (2011) The ecotoxicological and interactive effects of chromium and aluminum on growth, oxidative damage and antioxidant enzymes on two barley genotypes differing in Al tolerance. Env Exp Bot 70:185–191
Ali S, Farooq MA, Ysmeen T, Hussain S, Arif MS, Abbas F, Bharwana SA, Zhang G (2013) The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotox Environ Safe 89:66–72
Badawi GH, Yamauchi Y, Shimada E, Sasaki R, Kawano N, Tanaka K, Tanaka K (2004) Enhanced tolerance to salt stress and water déficit by overexpressing superoxide dismutase in tobacco (Nicotiana tabacum) chloroplasts. Plant Sci 166:919–928
Barros Júnior UD, Lima MDR, Barbosa MAM, Batista BL, Lobato AKS (2016) Biochemical responses of two species of Eucalyptus exposed to aluminum toxicity: oxidative stress and antioxidant metabolism. Not Bot Horti Agrobo 44:107–115
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Britez RM, Watanabe T, Jansen S, Reissmann CB, Osaki M (2002) The relationship between aluminium and silicon accumulation in leaves of Faramea marginata (Rubiaceae). New Phytol 156:437–444
Cakmak I, Horst WJ (1991) Effect of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227
Chen W, Liu P, Xu G, Cai M, Yu H, Chen M (2008) Effects of Al3+ on biological characteristics of cowpea root border cells. Acta Physiol Plant 30:303–308
Dallagnol LJ, Martins SCV, Matta FM, Rodrigues FA (2015) Brown spot negatively affects gas exchange and chlorophyll a fluorescence in rice leaves. Trop Plant Pathol 40:275–278
Demiate IM, Figueroa AM, Guidolin MEBZ, Santos TPR, Yangcheng H, Chang F, Jane J (2016) Physicochemical characterization of starches from dry beans cultivated in Brazil. Food Hydrocoll 61:812–820
Doncheva S, Poschenrieder C, Stoyanova Z, Georgieva K, Velichkova M, Barceló J (2009) Silicon amelioration of manganese toxicity in Mn-sensitive and Mn-tolerant maize varieties. Env Exp Bot 65:189–197
Dong B, Sang WL, Jiang X, Zhou JM, Kong FX, Hu W, Wang LS (2002) Effects of aluminum on physiological metabolism and antioxidant system of wheat (Triticum aestivum). Chemosphere 47:87–92
Dorneles AOS, Pereira AS, Rossato LV, Possebom G, Sasso VM, Bernardy K, Sandri RQ, Nicoloso FT, Ferreira PAA, Tabaldi LA (2016) Silicon reduces aluminum content in tissues and ameliorates its toxic effects on potato plant growth. Ciênc Rural 46:506–512
Dresler S, Wójcik M, Bednarek W, Hanaka A, Tukiendorf A (2015) The effect of silicon on maize growth under cadmium stress. Russ J Plant Physl 62:86–92
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
Giannakoula A, Moustakas M, Mylona P, Papadakis I, Yupsanis T (2008) Aluminum tolerance in maize is correlated with increased levels of mineral nutrients, carbohydrates and proline, and decreased levels of lipid peroxidation and Al accumulation. J Plant Physiol 165:385–396
Giannopolitis CN, Ries SK (1977) Superoxide dismutase I. Occurrence in higher plants. Plant Physiol 59:309–314
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gong M, Li YJ, Chen SZ (1998) Abscisic acid-induced thermotolerance in maize seedlings is mediated by calcium and associated with antioxidant systems. J Plant Physiol 153:488–496
Gu H, Zhan S, Wang S, Tang Y, Chaney RL, Fang X, Cai X, Qiu R (2012) Silicon-mediated amelioration of zinc toxicity in rice (Oryza sativa L.) seedlings. Plant Soil 350:193–204
Havir EA, McHale NA (1987) Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol 84:450–455
He G, Zhang J, Hu X, Wu J (2011) Effect of aluminum toxicity and phosphorus deficiency on the growth and photosynthesis of oil tea (Camellia oleifera Abel.) seedlings in acidic red soils. Acta Physiol Plant 33:1285–1292
Kidd PS, Llugany M, Poschenrieder C, Gunsé B, Barceló J (2001) The role of root exudates in aluminum resistance and silicon-induced amelioration of aluminium toxicity in tree varieties of maize (Zea mays L.). J Exp Bot 52:1339–1352
Li P, Song A, Li Z, Fan F, Liang Y (2015) Silicon ameliorates manganese toxicity by regulating manganese transport and antioxidant reactions in rice (Oryza sativa L.). Plant Soil 354:407–419
Liang Y, Sun W, Zhu Y, Christie P (2007) Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ Pollut 147:422–428
Lichtenthaler H, Buschmann C (2001) Chlorophylls and carotenoids: Measurement and characterization by UV–VIS spectroscopy. In: Wrolstad RE, Acree TE, An H, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Sporns P (eds) Current protocols in food analytical chemistry (CPFA) (Suppl 1). Wiley, New York
Lima MDR, Barros Júnior UO, Barbosa MAM, Segura FR, Silva FF, Batista BL, Lobato AKS (2016) Silicon mitigates oxidative stress and has positive effects in Eucalyptus platyphylla under aluminium toxicity toxicity. Plant Soil Environ 62:164–170
Ma JF, Yamaji N (2008) Functions and transport of silicon in plants. Cell Mol Life Sci 65:3049–3057
Ma JF, Mitani N, Nagao S, Konishi S, Tamai K, Iwashita T, Yano M (2004) Characterization of the silicon uptake system and molecular mapping of silicon transporter gene in rice. Plant Physiol 136:3284–3289
Ma JF, Yamaji N, Mitani-Ueno N (2011) Transport of silicon from roots to panicles in plants. Proc Jpn Acad B-Phys 87:377–385
Mali M, Aery NC (2008) Silicon effects on nodule growth, dry-matter production, and mineral nutrition of cowpea (Vigna unguiculata). J Plant Nutr Soil Sc 171:835–840
Matsumoto H, Motoda H (2013) Oxidative stress is associated with aluminum toxicity recovery in apex of pea root. Plant Soil 363:399–410
Mitani N, Ma JF (2005) Uptake system of silicon in different plant species. J Exp Bot 56:1255–1261
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Panda SK, Baluška F, Matsumoto H (2009) Aluminum stress signaling in plants. Plant Signal Behav 4:592–597
Pavlovic J, Samardzic J, Maksimovic V, Timotijevic G, Stevic N, Laursen KH, Hansen TH, Husted S, Schjoerring JK, Liang Y, Nicolic M (2013) Silicon alleviates iron deficiency in cucumber by promoting mobilization of iron in the root apoplast. New Phytol 198:1096–1107
Ribeiro MAQ, Almeida AF, Mielke MS, Gomes FP, Pires MV, Baligar VC (2013) Aluminum effects on growth, photosynthesis, and mineral nutrition of cacao genotypes. J Plant Nutr 36:1161–1179
Shen X, Xiao X, Dong Z, Chen Y (2014) Silicon effects on antioxidative enzymes and lipid peroxidation in leaves and roots of peanut under aluminum stress. Acta Physiol Plant 36:3063–3069
Singh VP, Tripathi DK, Kumar D, Chauhan DK (2011) Influence of exogenous silicon addition on aluminum tolerance in rice seedling. Biol Trace Elem Res 144:1260–1274
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant system in acid rain treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Wang Y, Stass A, Horst WJ (2004) Apoplastic binding of aluminum is involved in silicon-induced amelioration of aluminum toxicity in maize. Plant Physiol 136:3762–3770
Wu QS, Xia RX, Zou YN (2006) Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. J Plant Physiol 163:1101–1110
Yang L, Qi Y, Jiang H, Chen L (2013) Roles of organic acid anion secretion in aluminum tolerance of higher plants. Biomed Res Int 2013:1–16
Yang M, Tan L, Xu Y, Zhao Y, Cheng F, Ye S, Jiang W (2015) Effect of low pH and aluminum toxicity on the photosynthetic characteristics of different fast-growing Eucalyptus vegetatively propagated clones. PLoS One 10:1–16
Zhang C, Moutinho-Pereira JM, Correia C, Coutinho J, Gonçalves A, Guedes A, Gomes-Laranjo J (2013) Foliar application of Sili-K® increases chestnut (Castanea spp.) growth and photosynthesis, simultaneously increasing susceptibility to water deficit. Plant Soil 365:211–225
Zhou S, Sauve R, Thannhauser TW (2009) Aluminum induced proteome changes in tomato cotyledons. Plant Signal Behav 4:769–772
Acknowledgements
This research had financial supports from Fundação Amazônia de Amparo a Estudos e Pesquisas (FAPESPA/Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil) and Universidade Federal Rural da Amazônia (UFRA/Brazil) to Lobato AKS. While Jesus LR was supported by graduate scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Kovacik.
Electronic supplementary material
Below is the link to the electronic supplementary material.

11738_2017_2435_MOESM1_ESM.jpg
Supplementary material 1 (JPEG 11231 kb)Supplementary material 1. Greenhouse constructed in polycarbonate with temperature and humidity controlled (A), seedling emergence (B) seedling of cowpea (C) Jesus LR (author) changing nutritive solution (D), plants into greenhouse (E) and Lobato AKS (advisor) with other students (F). All image were not edited.

11738_2017_2435_MOESM2_ESM.jpg
Supplementary material 2 (JPEG 7819 kb) Supplementary material 2. Top view (A), side view (C) and leaf (E) from plants without Al and top view (B), side view (D) and leaf (F) from plants with 10 mM Al. All image were not edited.
Rights and permissions
About this article
Cite this article
de Jesus, L.R., Batista, B.L. & da Silva Lobato, A.K. Silicon reduces aluminum accumulation and mitigates toxic effects in cowpea plants. Acta Physiol Plant 39, 138 (2017). https://doi.org/10.1007/s11738-017-2435-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-017-2435-4