Abstract
Background and aims
Silicon (Si) was suggested to enhance plant resistance to toxic elements, and its beneficial role was mainly based on external and internal plant mechanisms. This work aimed at investigating the internal effect of Si on zinc (Zn) detoxification to rice (Oryza sativa L., cv. Tian You 116) seedlings.
Methods
In a hydroponic experiment, we examined the uptake, xylem loading and localization of Zn in rice seedlings under the condition of 200 μM Zn contamination with the additional silicate supply at three levels ( 0, 0.5 and 1.8 mM).
Results
The silicate addition significantly increased the seedling biomass, and decreased Zn concentration in both root and shoot of seedlings and in xylem sap flow. Zinpyr-1 fluorescence test and Energy-dispersive X-ray spectroscopy analysis showed the concentration of biologically active Zn2+ decreased, and Zn and Si co-localized in the cell wall of metabolically less active tissues, especially in sclerenchyma of root. The fractionation analysis further supported silicate supply increased about 10% the cell wall bound fraction of Zn.
Conclusions
This study suggests the Si-assisted Zn tolerance of rice is mainly due to the reduction of uptake and translocation of excess Zn, and a stronger binding of Zn in the cell wall of less bioactive tissues might also contribute to some degree.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc is commonly both a deficient and phytotoxic element in soils (Chaney 2010). It is an essential micronutrient for plant healthy growth and development. However, an excess of Zn presence in soil can be extremely toxic to plant cells by interfering with the uptake, transport and homeostasis of essential ions, and the disruption of metabolic processes. The greater mobility of Zn in the soils also contributes to the increasing concern of Zn phytotoxicity.
Silicon has not been considered as an essential mineral element for higher plants (Epstein 1999), but there is increasing evidence that Si is beneficial for healthy plant growth and development, particularly for rice in which SiO2 content accounts for about 10% of its dry shoot weight (Liang et al. 1994; Ma and Yamaji 2006). Silicon is also suggested to enhance the resistance to toxic elements, and the beneficial role of Si in detoxification could be concluded to be based on external (growth media) and internal plant mechanisms (Cocker et al. 1998; Wang et al. 2004). It has been found that the external plant mechanism of silicates ameliorative effect on toxic metals is mainly due to the decrease of metal phytoavailability by increasing pH (the Si-containing minerals most are alkaline) and forming metal silicate precipitates (Cocker et al. 1998; Ma et al. 1997). Such external effects are also supported by the in situ remediation investigations by adding amendments enriched with silicates (Chen et al. 2000; Dwivedi et al. 2007; Kumpiene et al. 2007; Rautaray et al. 2003; Rijkenberg and Depree 2010; Singh et al. 2008). On the other hand, the role of silicates in metal detoxification of plants was also found during recent years (Currie and Perry 2007; Epstein 2009; Gong et al. 2006; Liang et al. 2007). Silicate was reported to alleviate the manganese (Mn) toxicity by increasing the Mn fraction bound in cell wall (Iwasaki et al. 2002; Rogalla and Römheld 2002). Wang et al. (2004) showed that the apoplastic binding of aluminum (Al) was involved in Si-induced amelioration of Al toxicity in maize. Silicate has also been suggested to restrict the transport of cadmium (Cd) from roots to shoots in rice, because of the silicate deposition in the vicinity of the endodermis in roots (Shi et al. 2005). Silicates are also well known to be effective in alleviating arsenic (As) stress in rice (Bogdan and Schenk 2008; 2009; Guo et al. 2005; Ma et al. 2008). It has been shown that arsenite uptake by rice seedlings is affected by Si both external and internal plant (Guo et al. 2007). However, the mechanisms involved in the alleviation effect of Si on uptake of As are so far not clear.
From previous reports, it can be concluded that understanding of both external and internal plant effects of Si will be conducive to elaborating the Si-assisted metal resistance mechanisms. However, the majority of the work on Si effects on metal resistance of plant has been focused on the external mechanism (Chen et al. 2000; Kumpiene et al. 2007; Lee et al. 2006; Ma et al. 1997). The Si-induced amelioration mechanisms of plants were mainly on the tolerance of Al, Mn and Cd. A recent research reported that Si could alleviate Zn toxicity to rice by the Si-mediated antioxidant defense capacity and membrane integrity (Song et al. 2011). Whereas the research on the Zn resistance of plants was still few, especially on rice, typical Si-accumulator. The objectives of this work were to investigate: 1) the effect of Si on Zn uptake by rice seedlings; 2) the effect of Si on Zn translocation by xylem sap flow; 3) the effect of Si on Zn localization and transformation; and 4) the relationship of Zn and Si subcellular distribution in rice seedlings.
Materials and methods
Plant culture
The cultivar of rice (Oryza sativa L.) was Tian You 116, and the seeds were obtained from the Rice Research Institute of Guangdong Academy of Agricultural Sciences. They were surface-sterilized in 10% hydrogen peroxide for 20 min, washed with deionized water, then placed on four layers of filter paper to germinate at 30°C in darkness. Six days after germination, uniform seedlings were transferred into nutrient solution according to Kukier and Chaney (2002) in 2 L plastic pots (8 seedlings per pot) with some modifications. Because EDTA readily forms complexes with Zn2+ and affects Zn uptake, 10 μM FeSO4 replaced FeEDTA 2 days before Zn application and was added daily thereafter. The seedlings were firstly transplanted to 1/4 strength nutrient solution, 10 days later were changed to 1/2 strength solution, and then 6 days later the nutrient solution was changed to full-strength solution.
To investigate the influence of silicate on Zn phytotoxicity, seedlings were treated with additional silicate and Zn after culturing with full-strength solution for 3 days. Rice seedlings were exposed to the solutions with three additional levels of silicate (0, 0.5 and 1.8 mM, and represented by −Si, +Si0.5 and +Si1.8, respectively) affected by 0 and 200 μM additional concentrations of Zn (represented by –Zn and +Zn). The normal full-strength solution was as control. Seedlings were harvested for analysis after 40 days treatment. The concentrations of Si and Zn were 0.1 mM and 2 μM in normal nutrient solution. Silicon was supplied as silicic acid after neutralization of Na2SiO3 with HCl and Zn as ZnSO4. The pH of the nutrient solution was daily adjusted to 5.5 using NaOH or HCl, and the growth solution was renewed every 3 days.
Determination of Zn and Si in rice seedlings
After harvest, rice seedlings were washed with 10 mM ethylenediaminetetraacetic acid disodium salt (Na2EDTA, pH = 5.5) for 10 min and then three times with deionized water. The washed seedlings were separated into roots, stems + sheaths (the stems and sheaths were merged as one sample, because the biomass of stems was still little when the seedlings were harvested, and mainly wrapped by the sheath) and leaf blades. The dry weights of different parts of rice were measured. Dried tissue samples were ground with an electric steel mill to pass through a 1 mm sieve. The concentrations of Si and Zn in different parts of rice were determined. The test method of Si followed Elliott and Snyder (1991). The digestion method for measurement of Zn concentration followed Chen et al. (2004), and the digestion solutions were determined by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES, Optima 5300DV, Perkin-Elmer, USA).
Xylem-loading experiments
Prior to harvesting, the rice seedlings were decapitated 1 h after the nutrient solutions were renewed. The plants were decapitated 3–4 cm above the roots, and the detopped stalks were sealed with a silicone tube for collecting xylem exudates with a micropipette. The exudates were collected 3 h after decapitation. The Si concentration in the xylem sap was determined immediately according to the method of Liang et al. (2006) in order to avoid analytical errors caused by silicate polymerization, and the Zn concentration was measured by Graphite Furnace Atomic Absorption Spectrophotometer (GFAAS, Hitachi Z-2000, Japan).
Fluorescence microscopy study
Microscopic imaging of Zn in roots, sheaths and leaf blades of rice seedlings were conducted according to the method of Sinclair et al. (2007) with some modifications. Working solutions of Zn fluorescent indicator Zinpyr-1 (ZP-1, Sigma, Allentown, PA, USA) in 0.9% saline were diluted from 1 mM stock made up in dimethyl sulphoxide (DMSO) and stored at −20°C. After rice seedlings in control (without additional Si and Zn application) and treatments of +Zn-Si and +Zn +Si1.8 have grown for 40 days, rice seedlings were washed with 10 mM ethylenediaminetetraacetic acid disodium salt (Na2EDTA, pH = 5.5) for 10 min and then three times with deionized water. The washed fresh roots, sheaths and leaf blades of rice seedlings were cut into small pieces (thickness < 0.5 mm). All samples were then immersed in a 20 μM working solution of the ZP-1. The samples immersed in the solutions were firstly vacuum-infiltrated for 10 min, and incubated in the same solution for a further 20 min. Samples were then rinsed in deionized water. After washing, samples were mounted in 0.9% saline and images were taken on a fluorescent microscope (Zeiss Imager Z1, Germany) using filters S484/15 for excitation and S517/30 for emission. Exposure time was uniform for all samples. All images were taken at × 20 magnification. The plant sections without dye treating in control were also examined to observe the auto-fluorescence.
Scanning electron microscopy and energy dispersive X-ray analysis
The rice seedlings in treatments of –Zn +Si1.8, +Zn-Si and +Zn +Si1.8 were used. Fresh samples from the middle of roots, leaf sheaths and blades were rapidly frozen in liquid nitrogen and transversely cut into small segments of approximately 5 mm length using a pre-cooled knife. The procedures of sample preparation for microscopic scanning and energy dispersive analysis were reviewed by Shi et al. (2005). All the segments with smooth surfaces were freeze-dried at −80°C, and then coated with carbon. Carbon was used instead of a metal coating to avoid interference on the elements measured. The samples were observed under a thermal field-emission environmental scanning electron microscope (SEM, Model Quanta 400, FEI, Holand). Elemental analysis was undertaken using energy dispersive X-ray spectrometer (EDS, INCA, Oxford, Great Britain) at 20 kV.
Compartmental analysis of Zn in rice seedlings
Fresh rice seedlings grown in solutions of +Zn-Si and +Zn +Si1.8 for 40 days were used to analyze the Zn distribution in different compartmentation by the method of Rogalla et al. (Rogalla and Römheld 2002; Shi et al. 2005) with minor modifications. Rice seedlings were washed by following the same method used in fluorescence microscopy study, and then separated into fresh roots, stems + sheaths and leaf blades. All these plant tissues were then cut into the small pieces (thickness < 0.5 mm). The cut pieces were homogenized in 0.4 M hypertonic sucrose solution in a blender. The broken cells were recovered from the homogenate by centrifugation at speed 700 × g. To remove contaminant, cell wall was washed successively in increasing concentration of sucrose (0.4, 0.6, 1 M). The Zn in the washing sucrose solution was considered as the sum of Zn in free symplastic and free apoplastic space (FSFAS). Then the cell wall was washed successively with 0.1% Triton X-100 and deionized water, and the Zn in the washing solutions was considered as the sum of Zn in cytoplasm/vacuole. Finally the Zn bounded by cell wall were further fractionated by sequentially washing the residue of cell wall with BaCl2 (50 mM), DTPA (5 mM supplemented with 5 mM MES, pH 5.8) and HCl (0.5 mM) twice each. The Zn concentration in washing solutions was determined by GFAAS.
Zinc speciation calculations and statistical analysis
The chemical speciation of Zn in the exposure solutions was predicted using Visual MINTEQ (Version 2.53). All the values are the mean of 3 replicates in this study. Statistical analyses were performed with SPSS (Version 11.5). Differences among treatments were determined using Duncan’s test from the analysis of one-way variance (ANOVA) at a significance level of p < 0.05. Differences between treatments of +Zn-Si and +Zn +Si1.8 in compartmentation experiment were tested by independent-samples t test (p < 0.05).
Results
Species of Zn in exposure solutions
In the current study, the Visual MINTEQ program was used to evaluate the chemical speciation of Zn in the exposure solutions with the presence of silicate. The species of Zn in the solutions are shown in Table 1. The Zn present in the culture media regardless of in normal nutrient solution or treated by silicate was mainly comprised of the following species: free Zn2+, ZnSO4 (aq), ZnHPO4 (aq). The treatments of +Zn +Si0.5 and +Zn +Si1.8 did not significantly affect the activity of free Zn2+ in exposure solutions.
The growth of rice seedlings
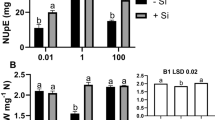
The addition of Zn (200 μM) significantly inhibited the growth of rice seedlings (Fig. 1). Although compared to control, the seedling biomass substantially improved only in higher external silicate (−Zn +Si1.8) treatment, the growth inhibition by Zn was markedly mitigated in both treatments with silicate, especially in +Zn +Si1.8 treatment, where up to 76% decrease of the seedling biomass was overcame. The dry weights of roots, sheaths + stems and leaf blades of seedlings in +Zn +Si1.8 treatment were 51%, 31% and 32% higher, respectively, than those in +Zn-Si treatment. The biomass of the whole plants was still 93% in +Zn +Si1.8 treatment, while only 82% in +Zn +Si0.5 treatment, compared to that in control.
Effects of silicate on the Zn accumulation and translocation in rice seedlings
The silicate supply decreased the Zn concentration in roots, stems + sheaths and leaf blades of rice seedlings stressed by Zn, especially in +Zn +Si1.8 treatment (Fig. 2). The Zn translocation in seedlings was also influenced by silicate addition (Table 2). The ratios of Zn contents in leaf blades to stems + sheaths decreased from 0.50 to 0.45 when silicate supply increased from 0 to 1.8 mM, which implied that Si application inhibited Zn transfer from stems + sheaths to leaf blades. Whereas, the ratios of Zn amount in stems + sheaths to roots did not show similar effect. Silicate addition changed the Zn distribution among different tissues of seedlings to some degree, especially in Zn +Si1.8 treatment where less Zn accumulated in leaf blade.
Zn in xylem exudates
The concentrations of Zn in xylem sap flow in +Zn +Si0.5 and +Zn +Si1.8 treatments significantly decreased, respectively, by 7.8% and 21% compared with +Zn-Si treatment (Table 3). This indicated that silicate addition inhibited Zn translocation by xylem flow.
ZP-1 fluorescence analysis
The use of the Zn-fluorophore, ZP-1, revealed the difference of Zn cellular localization across root, sheath and leaf blade cross-sections of rice seedlings in control, +Zn-Si and +Zn +Si1.8 treatments (Fig. 3). Strong Zn-dependent fluorescent signals were observed in sclerenchyma cell wall of root (Fig. 3-A4 and A4’), sheath (Fig. 3-B4 and B4’) and leaf blade (Fig. 3-C4 and C4’) cross-sections of seedlings in +Zn-Si treatment. The fluorescence intensity was also high in the endodermis and stele of roots (Fig. 3-A4”), and there were also fluorescence signals in cell wall of vascular bundle sheath and mesophyll of sheaths and leaf blades of seedlings exposed in +Zn-Si treatment. In contrast, the fluorescence intensity decreased in all root (Fig. 3-A3), sheath (Fig. 3-B3) and leaf blade (Fig. 3-C3) cross-sections of seedling growing in +Zn +Si1.8 solution. And only faint fluorescence was found in stele of roots, and in vascular bundle sheath of sheaths and leaf blades. There was no signal at all in mesophyll cells of either sheaths or leaf blades. The auto-fluorescence (Fig. 3-A1, B1 and C1) and background fluorescence (Fig. 3-A2, B2 and C2) were very slight in root, while a little strong in sclerenchyma tissues of sheath and leaf blade. However, we could still find the slight difference of fluorescence intensity in shoot between –Zn and +Zn treatments, especially at +Zn-Si condition. These results indicate that the concentration of free Zn2+ decreased in seedlings treated with 1.8 mM Si, especially in root, hence alleviated the toxicity of excessive Zn2+ to seedlings.
Fluoresce microscope images of roots, sheaths and leaf blades of rice seedlings in CK (without additional Si and Zn application), +Zn-Si and +Zn +Si1.8 treatments. The plant sections without dye treating in control were also examined to observe the auto-fluorescence. Images A4’, A4”, B4’ and C4’ were the local amplication of the parts in the white rectangle frames in image A4, B4 and C4, respectively. EX: exodermis; SC: sclerenchyma; EN: endodermis; C: cortex; AE: aerenchyma; S: stele; EP: epidermis; ENP: endepidermis; VBS: Vascular bundle sheath; V: vessel; ST: sieve tube; MC: mesophyll cell; UE: upper epidermis; LE: low epidermis
SEM-EDS analysis
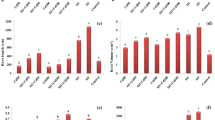
Figure 4 indicates the Zn and/or Si distribution in the cross-sections of roots, sheaths and leaf blades by line scans of SEM-EDS. In roots, the highest Si concentration was located around the endodermis, secondly around exodermis and sclerenchyma (Fig. 4-A1 and A3). The Si distribution showed similar patterns in –Zn +Si1.8 and +Zn +Si1.8 treatments. Zn is mainly deposited around exodermis and sclerenchyma of roots in +Zn-Si treatment (Fig. 4-A2). However, the distribution pattern of Zn was similar to that of Si in +Zn +Si1.8 treatment.
Distribution of Zn and/or Si in the cross-sections of roots, sheaths and leaf blades of rice seedlings in –Zn +Si1.8, +Zn-Si and +Zn +Si1.8 treatments. Image B3 was the local amplication of the part in the white rectangle frame in image B3’. The wavy lines showed the Kα line intensity of Zn and/or Si across the solid straight lines in the transverse sections. The EDS spectrums of root (A4), sheath (B4) and leaf blade (C4) in +Zn +Si1.8 treatment confirmed the presence of Zn and Si. EX: exodermis; SC: sclerenchyma; C: cortex; AE: aerenchyma; EN: endodermis; S: stele; EP: epidermis; ENP: endepidermis; V: vessel; ST: sieve tube; MC: mesophyll cell; UE: upper epidermis; LE: low epidermis
In leaf sheaths, the preferential localization of Si was around epidermis and sclerenchyma of in both –Zn +Si1.8 and +Zn +Si1.8 treatments. In leaf blade sections, Si was mainly distributed in upper and lower epidermis. More clear Zn distribution variability in shoot could not be given by EDS in this study because of the low amount presented. The local amplication revealed that Zn concentration was a little higher in sclerenchyma cells of leaf sheath where Si was also highly accumulated (Fig. 4-B2 and B3).
The Zn distribution in different compartments of rice seedlings
The compartmental analysis showed that, compared with +Zn-Si treatment, +Zn +Si1.8 treatment yielded less Zn distribution in the free symplastic and free apoplastic space and cytoplasm/vacuole of seedling roots, stems + sheaths and leaf blades (Table 4). However, in +Zn +Si1.8 treatment, more Zn was found to be bonded to the cell wall by 12% increase in roots, 9.1% in stems + sheaths and 9.9% in leaf blades. This suggested that silicate addition changed the compartment distribution of Zn, and increased the cell-wall-bound fraction in the whole seedling plant.
Discussion
The ameliorative effects of Si on metal toxicity to plants were attributed to an external plant effect by decreasing availability of phytotoxic metals in the growth media (da Cunha et al. 2008; Liang et al. 2005). The decrease in metal concentrations in plant could be induced by the formation of biologically inactive complexes of metal silicates in soils. In this study, compared with +Zn-Si treatment, the treatments of +Zn +Si0.5 and +Zn +Si1.8 did not significantly affect the activity of free Zn2+ calculated by the Visual MINTEQ program in exposure solutions with 200 μM Zn. However, the silicate amended treatments reduced the inhibitory effects of excessive Zn on seedling growth with plant biomass increment, especially when sufficient silicate was supplied. This result is consistent with the report that the Al toxicity amelioration was obtained in maize with no hydroxyaluminumsilicate formation in culture solution (Wang et al. 2004). In current study, it was also found that there was no difference of the total amount of Zn in seedlings regardless of with or without silicate application. However, the distribution of Zn in different tissues changed by silicate treatment. Further, silicate supply decreased Zn transfer from stems + sheaths to leaf blades, especially in high silicate treatment. The similar phenomenon of metal translocation inhibition by Si was also found in our soil culture experiment where Si-rich amendments were used (Gu et al. 2011). Song et al. (2011) also reported that Si supply decreased the Zn translocation from root to shoot. Therefore, we consider an internal plant effect as the main contributing factor to the amelioration of excessive Zn toxicity by silicate under the conditions of present experiment.
The transformation of free metal ions into nonphytotoxic forms could effectively minimize the damage from excess exposure (Cocker et al. 1998; Hodson and Sangster 1999). The transformation and subcellular localization of Zn2+ was studied to identify the effects of Si on Zn detoxification. The fluorescent dye ZP-1 is useful for intracellular work because it is membrane permeable and highly selective for Zn2+ over other biological metal ions, and metal-dependent fluorescence occurs only upon binding to biologically active Zn2+ or Cd2+ (Burdette et al. 2001). No Cd was present in current experiment; hence the ZP-1-dependent fluorescence in seedlings is influenced by exogenous Zn2+ and silicate effects. Although there were auto-fluorescence and background fluorescence in sclerenchyma tissues of shoot, in both the treatments of +Zn-Si and +Zn +Si1.8, the stronger fluorescent was found in the cell wall of sclerenchyma, endodermis and stele of the root, sclerenchyma and vascular bundle of the sheath and leaf blade. This might be a consequence of those tissues, especially sclerenchyma tissues in root, with thick cell wall which sequestrated more Zn2+ through the barrier/binder function. In the presence of silicate, the general staining of Zn with ZP-1 was less intense in seedlings than that treated with Zn alone, which suggested that silicate addition reduced the concentration of biologically active Zn2+ in seedlings. While more sensitive method was recommended for a more clear analysis of Zn distribution and transformation in rice shoot in the future. Here, the intensity of the fluorescence provides only a qualitative comparison, since the fluorescence response of the dye is usually not linear, but rather a signal function of the metal concentration (Tian et al. 2009). The decrease of fluorescence in Si-amended treatment might be a result of the silicate detoxification effects, the possible explanations are as follows: On one hand, the silicate supply strengthened the cell wall, thus inhibited the Zn2+ translocation (Currie and Perry 2007; Gong et al. 2006; Huang et al. 2009; Peleg et al. 2010). On the other hand, the heavy deposition and incorporation of silicates within the plant cell wall because of the biochemical components (Currie and Perry 2007) increased the Zn2+ binding sites, which might induce the transformation from Zn2+ into depositions. It has been widely accepted that sequestration of metals in metabolically less active cell compartments, such as the cell wall of support tissue, plays an important role in accumulating and detoxifying metals before they reach more sensitive site, especially the mesophyll (da Cunha and do Nascimento 2009; Hodson and Sangster 1999; Neumann et al. 1997; Shi et al. 2005). Silicon improved seedlings biomass and diluted the total Zn concentration also contributed to that to some degree.
EDS was used to further clarify the distribution of Zn and the interaction with Si in rice seedlings. The EDS spectra show that distribution pattern of Zn was changed in roots by silicate addition. What is more interesting is that the distribution of Zn and Si resemble to some degree, and both of them preferentially deposited around endodermis of roots. The possible reason for this phenomenon is that the higher silicate could provide more metal ions binding sites in plant, which induced the combination or chelation between silicate and metal ions (Wang et al. 2000). The formation of Zn-Si precipitates is a possible reason for the lower Zn concentration in xylem sap flow in the silicate amended treatments, because the heavy deposition of Zn and Si in endodermis might partially restrain xylem loading of Zn, thus restrained Zn transport. This is consistent with the report of da Cunha and do Nascimento (2009) that the addition of silicate reduced the metals apoplastic transport by deposition in the endodermis cell wall. The co-location of Zn and Si seems to occur not only in root but also possibly in aerial part, and it could be found that the heavier distribution in sclerenchyma of leaf sheath, which might contribute to the ratio reduction of Zn accumulation in leaf blades to stems + sheaths in this study. The Zn distribution detected by EDS was consistent with the result of ZP-1 fluorescence in present study. Hence it further strengthened our above deduction that the formation of Zn-Si complexes in less active parts is responsible for the Zn detoxification and sequestration. However, the in-depth studies of mechanisms underlying the Si-assisted toxic metal tolerance in rice, especially in shoot, needed more sensitive methods such as synchroton radiation X-ray fluorescence (SRXRF) analysis.
The ZP-1 and SEM-EDS techniques cannot provide quantitative information on Zn localization. Therefore, a fractionation technique was used in this study. The results showed the silicate addition increased the cell-wall-bound fraction of Zn in all tissues of seedlings including roots, stems + sheaths and leaf blades. The report about cucumber, which accumulates high levels Si in the tops, also supported the role of Si in Mn tolerance, and it found more Mn was bound to the cell wall in silicate amended treatment (Rogalla and Römheld 2002). Neumann et al. (1997) reported Zn was co-precipitated as Zn silicates in the leaf epidermal cell wall of Minuartia verna, a Si-accumulating dicotyledon, and this was an explanation of high Zn tolerance in this plant. However, it is unlikely that a mechanism of Si-assisted binding of metals to the cell wall is a general phenomenon in Si-accumulating plants. It was reported that silicate application did not increase cell wall bound fraction of Al in maize (Wang et al. 2004). Shi (2005) also found that Si did not remarkably change the distribution ratio of Cd in symplasm and apoplast of rice. The mechanisms of Si-assisted plant tolerance to high metal concentrations differ greatly, which might be associated with plant species, metal elements, and so on.
Conclusions
Under conditions of the present study, the ameliorative mechanism of silicate on excessive Zn toxicity to rice could be attributed to an internal plant effect. Because of the reduction of Zn concentration in xylem exudates and in both root and shoot by silicate treatments, the main ameliorative effect is the reduction of both uptake and translocation of excess Zn. The possible effect of silicate on the formation of Zn-Si precipitates and the transformation of Zn2+ into non-phytotoxic forms in the cell wall of less bioactive tissues, especially in sclerenchyma of root, could also be considered. However, a detailed understanding of the silicate effects on metal localization and transformation is still lacking, hence further studies are necessary for metal detoxification in rice.
References
Bogdan K, Schenk MK (2008) Arsenic in rice (Oryza sativa L.) related to dynamics of arsenic and silicic acid in paddy soils. Environ Sci Technol 42:7885–7890
Bogdan K, Schenk MK (2009) Evaluation of soil characteristics potentially affecting arsenic concentration in paddy rice (Oryza sativa L.). Environ Pollut 157:2617–2621
Burdette SC, Walkup GK, Spingler B, Tsien RY, Lippard SJ (2001) Fluorescent sensors for Zn2+ based on a fluorescein platform: Synthesis, properties and intracellular distribution. J Am Chem Soc 123:7831–7841
Chaney RL (2010) Cadmium and zinc. In: Hooda PS (ed) Trace Elements in Soils. Blackwell Publisher, Oxford, UK, pp 409–439
Chen HM, Zheng CR, Tu C, Shen ZG (2000) Chemical methods and phytoremediation of soil contaminated with heavy metals. Chemosphere 41:229–234
Chen YX, Shi JY, Zhang WD, Lin Q, Tian GM (2004) EDTA and industrial waste water improving the bioavailability of different Cu forms in contaminated soil. Plant Soil 261:117–125
Cocker KM, Evans DE, Hodson MJ (1998) The amelioration of aluminium toxicity by silicon in higher plants: Solution chemistry or an in planta mechanism? Physiol Plantarum 104:608–614
Currie HA, Perry CC (2007) Silica in plants: Biological, biochemical and chemical studies. Ann Bot 100:1383–1389
da Cunha KPV, do Nascimento CWA (2009) Silicon effects on metal tolerance and structural changes in maize (Zea mays L.) grown on a cadmium and zinc enriched soil. Water Air Soil Pollut 197:323–330
da Cunha KPV, do Nascimento CWA, Dda Silva AJ (2008) Silicon alleviates the toxicity of cadmium and zinc for maize (Zea mays L.) grown on a contaminated soil. J Plant Nutr Soil Sci 171:849–853
Dwivedi S, Tripathi RD, Srivastava S, Mishra S, Shukla MK, Tiwari KK, Singh R, Rai UN (2007) Growth performance and biochemical responses of three rice (Oryza sativa L.) cultivars in fly-ash amended soil grown. Chemosphere 67:140–151
Elliott CL, Snyder GH (1991) Autoclave-induced digestion for the colorimetric determination of silicon in rice straw. J Agr Food Chem 39:1118–1119
Epstein E (1999) Silicon. Annu Rev Plant Physiol Plant Mol Biol 50:641–664
Epstein E (2009) Silicon: its manifold roles in plants. Ann Appl Biol 155:155–160
Gong HJ, Randall DP, Flowers TJ (2006) Silicon deposition in the root reduces sodium uptake in rice (Oryza sativa L.) seedlings by reducing bypass flow. Plant Cell Environ 29:1970–1979
Gu HH, Qiu H, Tian T, Zhan SS, Deng THB, Chaney RL, Wang SZ, Tang YT, Morel JL and Qiu RL (2011) Mitigation effects of silicon rich amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on multi-metal contaminated acidic soil. Chemosphere doi:10.1016/j.chemosphere.2011.03.014
Guo W, Hou YL, Wang SG, Zhu YG (2005) Effect of silicate on the growth and arsenate uptake by rice (Oryza sativa L.) seedlings in solution culture. Plant Soil 272:173–181
Guo W, Zhu YG, Liu WJ, Liang YC, Geng CN, Wang SG (2007) Is the effect of silicon on rice uptake of arsenate (As-v) related to internal silicon concentrations, iron plaque and phosphate nutrition? Environ Pollut 148:251–257
Hodson MJ, Sangster AG (1999) Aluminium/silicon interactions in conifers. J Inorg Biochem 76:89–98
Huang CF, Yamaji N, Nishimura M, Tajima S, Ma JF (2009) A Rice Mutant Sensitive to Al Toxicity is Defective in the Specification of Root Outer Cell Layers. Plant Cell Physiol 50:976–985
Iwasaki K, Maier P, Fecht M, Horst WJ (2002) Effects of silicon supply on apoplastic manganese concentrations in leaves and their relation to manganese tolerance in cowpea (Vigna unguiculata (L.) Walp.). Plant Soil 238:281–288
Kukier U, Chaney RL (2002) Growing rice grain with controlled cadmium concentrations. J Plant Nutr 25:1793–1820
Kumpiene J, Lagerkvist A, Maurice C (2007) Stabilization of Pb- and Cu-contaminated soil using coal fly ash and peat. Environ Pollut 145:365–373
Lee H, Ha HS, Lee CH, Lee YB, Kim PJ (2006) Fly ash effect on improving soil properties and rice productivity in Korean paddy soils. Bioresour Technol 97:1490–1497
Liang YC, Sheng T, Li FJ, Feng YJ (1994) Silicon availability and response of rice and wheat to silicon in calcareous soils. Commun Soil Sci Plant 25:2285–2297
Liang YC, Wong JWC, Wei L (2005) Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 58:475–483
Liang YC, Hua HX, Zhu YG, Zhang J, Cheng CM, Romheld V (2006) Importance of plant species and external silicon concentration to active silicon uptake and transport. New Phytol 172:63–72
Liang YC, Sun WC, Zhu YG, Christie P (2007) Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ Pollut 147:422–428
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11:392–397
Ma JF, Sasaki M, Matsumoto H (1997) Al-induced inhibition of root elongation in corn, Zea mays L. is overcome by Si addition. Plant Soil 188:171–176
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. P Natl Acad Sci USA 105:9931–9935
Neumann D, zur Nieden U, Schwieger W, Leopold I, Lichtenberger O (1997) Heavy metal tolerance of Minuartia verna. J Plant Physiol 151:101–108
Peleg Z, Saranga Y, Fahima T, Aharoni A, Elbaum R (2010) Genetic control over silica deposition in wheat awns. Physiol Plantarum 140:10–20
Rautaray SK, Ghosh BC, Mittra BN (2003) Effect of fly ash, organic wastes and chemical fertilizers on yield, nutrient uptake, heavy metal content and residual fertility in a rice-mustard cropping sequence under acid lateritic soils. Bioresour Technol 90:275–283
Rijkenberg MJA, Depree CV (2010) Heavy metal stabilization in contaminated road-derived sediments. Sci Total Environ 408:1212–1220
Rogalla H, Römheld V (2002) Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumis sativus L. Plant Cell Environ 25:549–555
Shi XH, Zhang CC, Wang H, Zhang FS (2005) Effect of Si on the distribution of Cd in rice seedlings. Plant Soil 272:53–60
Sinclair SA, Sherson SM, Jarvis R, Camakaris J, Cobbett CS (2007) The use of the zinc-fluorophore, Zinpyr-1, in the study of zinc homeostasis in Arabidopsis roots. New Phytol 174:39–45
Singh A, Sharma RK, Agrawal SB (2008) Effects of fly ash incorporation on heavy metal accumulation, growth and yield responses of Beta vulgaris plants. Bioresour Technol 99:7200–7207
Song AL, Li P, Li ZJ, Fan FL, Nikolic M, Liang YC (2011) The alleviation of zinc toxicity by silicon is related to zinc transport and antioxidative reactions in rice. Plant Soil. doi:10.1007/s11104-011-0749-3
Tian SK, Lu LL, Yang XE, Labavitch JM, Huang YY, Brown P (2009) Stem and leaf sequestration of zinc at the cellular level in the hyperaccumulator Sedum alfredii. New Phytol 182:116–126
Wang LJ, Wang YH, Chen Q, Cao WD, Li M, Zhang FS (2000) Silicon induced cadmium tolerance of rice seedlings. J Plant Nutr 23:1397–1406
Wang YX, Stass A, Horst WJ (2004) Apoplastic binding of aluminum is involved in silicon-induced amelioration of aluminum toxicity in maize. Plant Physiol 136:3762–3770
Acknowledgements
The project was supported by the NSFC-Guangdong Joint Foundation of China (No. U0833004), Guangdong Provincial Natural Science Foundation (No. 06202438), National High Technology Research and Development Program of China (863 Program) (No. 2007AA06Z305 and 2007AA061001) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jian Feng Ma.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Gu, HH., Zhan, SS., Wang, SZ. et al. Silicon-mediated amelioration of zinc toxicity in rice (Oryza sativa L.) seedlings. Plant Soil 350, 193–204 (2012). https://doi.org/10.1007/s11104-011-0894-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0894-8