Abstract
The objective of this study was to analyze the mechanism of some physiological processes accompanying acquisition of sunflower (Helianthus annuus L.) chilling resistance due to seeds hydropriming in the presence of salicylic acid, jasmonic acid, 24-epibrassinolide followed exposition of seeds to short-term heat shock treatment. The seeds were hydroprimed at 25 °C in limited amounts of water or solution of salicylic or jasmonic acid at 10−2, 10−3 and 10−4 M concentration, 24-epibrassinolide at 10−6, 10−8 and 10−10 M concentration. The seeds were incubated for 2 days, subjected to short-term heat shock (45 °C, 2 h) and chilled for 21 days at 0 °C. Sunflower chilling susceptibility and physiological responses were evaluated according to the inhibition of radicle growth, the inhibition of the number of lateral roots formation, the activity of catalase and changes in soluble carbohydrates in seedlings developing for 72 h at 25 °C. Hydropriming and short-term heat shock application explicitly reduced inhibition of roots as well as lateral roots development by allowing the germinating seeds to recover from the growth-inhibiting effects of chilling. Seeds hydropriming in solutions containing salicylic acid, jasmonic acid and 24-epibrassinolide followed heat shock treatment additionally promoted the activity of catalase and sugars metabolism, which stimulated seedlings development and alleviated the decrease of F v/F m caused by chilling conditions. These beneficial effects contributed to increased resistance of sunflower seedlings to chilling stress. The present study demonstrated that the most profitable effect on reducing negative effect of chilling may be achieved by short-term heat shock applied during hydropriming in water supplemented with 24-epiBL (10−8 and 10−10 M) or salicylic acid (10−3 and 10−4 M).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sunflower (Helianthus annuus L.), a plant species native to North America, is one of the major oil seed crops in the world. It is also a good source of proteins comprising about 15–20% of the whole seed and 40% of the dehulled oil meal (Balasaraswathi and Sadasivam 1997). Owing to its high adaptability (relatively high tolerance to drought, insensitive to photoperiod), it is grown in many countries with the world harvested area of above 25.2 million hectares and the seed production about 41.4 million tons (FAOSTAT 2014). Even, in Poland, where the climate is colder than its thermal requirements, sunflower yields more than any other summer oil crops. However, the sunflower cultivation for edible oil is very limited, because usually ripens at autumn conditions with low temperatures, the dew in the morning or evening and high relative humidity. This inhibits the natural drying of plants after achenes maturation. Moving the sowing date earlier would allow avoiding unfavorable weather conditions during autumn and extending the growing season, thereby increasing seed yields of high quality (Hewezi et al. 2006). However, seedlings derived from earlier sowing are usually exposed to prolonged low or/and fluctuating/unstable temperatures during juvenile stages of sunflower development. Consequently, it may greatly delay seedlings emergence, development, establishment, and cause serious chilling injuries as well as infection diseases resulting in a significant reduction in yield and low seed quality. Chilling may also induce diverse phenotypic and physiological symptoms, leading to oxidative stress and loss of membrane integrity, which may disrupt general metabolic processes (Pál et al. 2013). Moreover, the exposure to low temperature has harmful effect on photosynthesis and photosystem II (PS II) which is especially susceptible to injuries. Decreased photochemical efficiency of photosystem II due to negative chilling effect is frequently expressed as variable to maximum fluorescence ratio (F v/F m) (Zhou and Guo 2009). As a consequence of chilling stress, the excess of excitation energy is not promptly quenched and in turn accelerates the formation of reactive oxygen species (ROS), resulting in the reduction of the photosynthetic efficiency (Zhang et al. 2010).
Such obstacles have increased the importance of sunflower tolerance to chilling (non-freezing) temperatures. Chilling is considered as one of the most important environmental factors negatively influencing plant establishment and performance. However, plants developed protecting mechanisms against chilling stress. Recent findings showed that plants can adapt to chilling conditions due to low temperature pretreatments (Zhang et al. 2017). Apparently, such treatments induced series of physiological and molecular responses which in turn led to increased chilling tolerance. Kang and Saltveit (2001) applied some methods to alleviate chilling injuries. Among them are seedling pretreatments with low pressure, high relative humidity, increased levels of atmospheric carbon dioxide, calcium, ethylene or extreme temperatures (i.e., heat or cold shock). These methods were mainly used during initial growth of plants. Therefore, it is difficult to apply them in practice. It would be a great interest to assess whether the beneficial responses of the methods could be applied during sunflower seed imbibition prior radicle protrusion.
Some of plant hormones, like 24-epibrassinolide, salicylic and jasmonic acid are involved in plant responses to stresses and take part in alleviating the negative effects of stress on plants. Brassinosteroids are the plant steroids which have been implicated in protecting plants from various types of stresses like chilling, heat, drought, salt, heat and heavy metals (Górnik et al. 2014). Salicylic acid (SA), a stress-related signaling compound, may directly or indirectly mediate local and systemic defense responses against pathogens and play a role in plant responses to abiotic stresses including drought, low and high temperatures, heavy metals, and osmotic stress (Janda et al. 2014). Jasmonic acid (JA) participates in various processes of plant establishment and performance, like germination of seeds, growth of callus and primary root, flowering, formation of gum and bulb and senescence (Fahad et al. 2014). Moreover, JA is involved in plant protection against biotic and stresses, such as insect wounding, attack by various pathogens, drought, chilling and salinity (Fahad et al. 2014).
The objective of this study was to analyze the effect of sunflower (H. annuus L.) seeds hydropriming in the presence of 24-epibrassinolide (24-epiBL), salicylic or jasmonic acid followed exposition of seeds to short-term heat shock on the seedling’s resistance to chilling stress and changes in soluble carbohydrates.
Materials and methods
Plant material
Experiments were carried out with seeds of sunflower (H. annuus L. cv. Wielkopolski). The seeds were purchased from HR Strzelce Sp. z o.o. company (Borowo, Poland). Seeds until the start of experiments were held at 15 °C and 30% of relative humidity for at least 9 months.
Seed hydropriming with phytohormones and heat shock treatment
The whole seeds (with pericarp) of sunflower were hydroprimed at 25 °C to 15% moisture content in limited amounts of water or solutions of 24-epibrassinolide (24-epiBL) at 10−6, 10−8 and 10−10 M concentration, salicylic acid (SA) or jasmonic acid (JA) at 10−2, 10−3 and 10−4 M concentration. The seeds were incubated for 2 days in air-tight glass bottles at 25 °C. During the incubation, the seeds were aired (ventilated) every day for about 15 s. After incubation, the seeds were subsequently subjected to short-term (2 h) heat shock (45 °C) and transferred to 6-cm Petri dishes containing a wet paper filters and incubated at 25 °C. The seedlings with 5 mm length were transferred to Phytotox kit Microbiotest, chilled for 21 days (at 0 °C). The activity of catalase was assayed in chilled seeds. The ability of seeds to survive chilling stress was assayed by the measurement of the length of root and number of developing lateral roots after 72 h of seeds germination at 25 °C (recovery). Soluble carbohydrates were analyzed in root with hypocotyl and cotyledons of seedlings after recovery from chilling stress. As a control, seeds without hydropriming and heat shock treatment were used.
The temperature treatments were carried in the following experimental protocol:
25 °C (2 days—incubation) → 45 °C (2 h—short-term heat shock) → 25 °C (≈2 days—till seedling roots reached 5 mm length) → 0 °C (21 days—chilling period) → 25 °C (72 h—recovery).
Measurement of seedlings development
Sunflower chilling susceptibility was measured as the inhibition of subsequent radicle elongation at 25 °C for 72 h after chilling of imbibed seeds at 0 °C for 21 days. The inhibition of radicle growth was evaluated as a percentage of non-chilled control. In the same manner was measured the inhibition of the number of lateral roots formation.
Catalase activity
Catalase activity was assayed spectrophotometrically as the reduction in absorbance at 240 nm due to the decline of extinction of H2O2, according to the procedure by Bailly et al. (1996) with some modifications. The test was carried out in a total volume of 3 mL of 50 mM potassium phosphate buffer (pH 7.0) containing 37.5 mM of H2O2 and 0.2 mL of enzyme extract. The UVmini-1240 spectrophotometer (SHIMADZU, Japan) and software for measuring kinetics (Kinetics Program Pack for UVmini-1240) of absorbance monitored for 2 min were applied. The reaction was started by addition of H2O2. The catalase activity was expressed as nanomoles of H2O2 decomposed per minute per mg of protein and corresponds to nine measurements carried out on three different extracts (three measurements per extract).
Chlorophyll fluorescence of intact leaf
After hydropriming of seeds in the solutions of phytohormones with heat shock application, the seedlings with 5-mm root length were sown in the pots and subjected to chilling conditions for 21 days at 0 °C. After transferring of seedlings to conditions of 25 °C for 2 weeks (recovery), the measurements of the efficiency of photosystem II (PSII) were carried out on fully expanded leaves using a pulse-modulated Fluorescence Monitoring System (FMS-1; Hansatech Instruments Ltd., Norfolk, UK) operated in the F v/F m mode according to the manufacturer’s instruction. Before each measuring time, the plant leaves were dark adapted for at least 20 min to allow all reaction centers to open and minimize fluorescence associated with the energization of the thylakoid membrane. The fiber optic of the FMS-1 was positioned using the PPF/temperature leaf clip at a 60° angle from the upper surface of the leaf, and the distance between the fiber optic and the leaf surface was kept constant for all measurements. The parameters of chlorophyll fluorescence: maximum fluorescence (F m), and variable fluorescence F v(F m − F o), were measured in the dark-adapted leaves using leaf clips. Initial fluorescence (F o) was measured at PPFD <0.05 μmol m−2 s−1, following by a saturating pulse to determine the maximum fluorescence emission in the absence (F m) of quenching was 3000 mmol m−2 s−1. The intensity of saturation pulses to determine the maximum fluorescence emission in the absence (F m) of quenching was 1800 mmol m−2 s−1. The efficiency of PSII photochemistry (F v/F m) in the dark-adapted state was considered as a useful measurement of photosynthetic performance of plants and as stress indicators. Maximum photochemical efficiency of PSII was estimated by the equation: F v/F m = (F m − F o)/F m.
Soluble carbohydrate analysis
Soluble carbohydrates were analyzed in seedlings after 72 h of recovery from chilling stress. For analyses were used control seedlings (developing from dry seeds without conditioning before chilling stress) and seedlings developing from seeds conditioned in water, SA (at 10−3M), JA (at 10−3M) and 24-epiBL (at 10−8M). Tissues of radicle (including hypocotyl) and cotyledons (separately) were frozen in liquid nitrogen and stored at −80 °C for a week. After lyophilization, dry tissues were thin milled using a mixer mill (MM200, Retsch, Verder Group, Netherlands) with a 20-Hz vibrational frequency for a 1.5 min. Soluble carbohydrates were extracted from 45 to 50 mg of meal with 450 µL of mixture of ethanol:water (1:1, v/v, containing 100 µg of xylitol as internal standard) and 450 µL of chloroform. Samples were incubated at 70 °C for 20 min (with still shaking, 1000 rpm, Thermo-Shaker, Aosheng, China). Homogenate was centrifuged (14,000g at 4 °C for 20 min) and 300 µL of upper layer (methanol:water) was transferred into 1.5-mL eppendorf tube containing 200 µL of mixture of ion-exchanger resins (DOWEX, SIGMA, USA). After shaking (1300 rpm for 45 min, Genie 2, Scientific Industries, USA) and centrifugation, an aliquot of supernatant (200 µL) was evaporated to dryness in a centrifugal vacuum concentrator (JW Electronic, Poland). Carbohydrates were derivatized with a mixture of trimethylsilyl imidazole: pyridine (1:1, v/v) at 75 °C for 45 min. Trimethylsilyl derivatives of soluble carbohydrates were analyzed by capillary gas chromatography method, as described previously (Lahuta 2006). Carbohydrates were quantified using original standards of sugars (Sigma, USA). Carbohydrates content was calculated from standard curves of appropriate component and results of analyses are means of three independent replicates ±SE.
Statistical analyses
The experiment was conducted three times. The measurements of radicles and the number of lateral roots were designed in four replications, each comprising ten seedlings. Catalase activity and chlorophyll fluorescence were repeated four times. The differences between the means were estimated by the Duncan multiple range test at a significance level of P = 0.05. In the case of soluble carbohydrate, the significance of differences in the sugars concentration was tested by ANOVA and Tukey’s post test (if overall P < 0.05) for multiple comparisons.
Results
The beneficial effects of seeds conditioning on chilling tolerance revealed already after 72 h of recovery. Roots of control seedlings reached length ca. 24 mm, whereas roots of seedlings developing from seeds conditioned in water elongated twofold faster (up to 52 mm, not presented data). The presence of phytohormones in conditioning solutions additionally increased seeds chilling resistance, according to the type and concentration of phytohormone used.
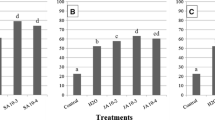
The exposition of imbibed seeds to chilling (for 21 days at 0 °C) inhibited subsequent elongation of radicles by 79.3%, as compared to non-chilled control (Fig. 1). Short-term heat shock applied together with hydropriming in H2O significantly limited (to about 52.2%) inhibitory effect of chilling on radicles elongation. Phytohormones (presented in priming solutions during these treatments) additionally reduced inhibition of radicle growth caused by chilling conditions. The most pronounced results were obtained after application of 24-epiBL at 10−8 or 10−10 M concentration (Figs. 1, 3). Due to such treatment, the inhibition of radicle elongation was reduced to 20.6 and 23.9%, respectively. Salicylic acid (SA) and jasmonic acid (JA) applied at 10−3 M concentration reduced chilling-induced inhibition of radicle elongation to 27.6 and 42.2, respectively.
Inhibition of radicle elongation (as a percentage of non-chilled and non-heat shocked seedlings) of sunflower seedlings caused by chilling stress (21 days at 0 °C). Seeds before chilling were hydroprimed in distilled water (H2O) or solutions of a salicylic acid (SA, at 10−2, 10−3 and 10−4 M) or b jasmonic acid (JA, at 10−2; 10−3 and 10−4 M) or c 24-epibrassinolide (24-epiBL, at 10−6, 10−8 and 10−10 M) for 2 days at 25 °C (to 15% moisture content) and then heat shocked (2 h at 45 °C) and returned to 25 °C for further growth to reach 5 mm of root length. Such seedlings were chilled for 21 days at 0 °C. Radicle elongation was measured after 72 h of recovery (25 °C) from chilling stress. As a control were used seeds without hydropriming (and non-heat shocked) and chilled at 0 °C for 21 days. Means with the same letter are not significantly different at P = 0.05 according to the Duncan multiply range test (n = 30)
Chilling conditions also remarkably inhibited (to 70.3%) lateral roots formation (Fig. 2). Both short-term heat shock exposure and phytohormones (applied during seed hydropriming) alleviated inhibitory effect of chilling stress on lateral root development. The most beneficial effect was obtained in the presence of SA (Figs. 2, 3). Applied in concentration of 10−4 M reduced the inhibition of lateral roots development caused by chilling to 12%. Similarly, 24-epiBL at 10−10 M significantly decreased chilling-induced inhibition of lateral roots development (25.9%). Application of JA (at 10−4 M) resulted in 42.4% reduction of the chilling-induced inhibition of lateral roots development.
Inhibition of the number of lateral roots development (as a percentage of non-chilled and non-heat shocked seedlings) of sunflower seedlings caused by chilling stress. Seeds treatment before stress was described on Fig. 1
Seedlings of sunflower derived from seeds hydroprimed in solutions of a salicylic acid (SA, at 10−2, 10−3 and 10−4 M) or b jasmonic acid (JA, at 10−2, 10−3 and 10−4 M) or c 24-epibrassinolide (24-epiBL, at 10−6, 10−8 and 10−10 M) for 2 days at 25 °C (to 15% moisture content) and then heat shocked (2 h at 45 °C) and returned to 25 °C for further growth to reach 5 mm of root length. Such seedlings were chilled for 21 days at 0 °C. The seedlings marked as “Chilled” derived from seeds moistened in distilled water without hydropriming (and non-heat shocked) and then chilled for 21 days at 0 °C. The presented picture were took after 72 h of recovery (25 °C) from chilling stress
Low temperature exposure (for 21 days at 0 °C) reduced catalase activity (CA) in control chilled seeds to 0.14 nmol H2O2 min−1 mg protein−1 (Fig. 4). Heat shock applied during seed hydropriming reduced chilling-induced inhibition of CA activity to 0.17 nmol H2O2 min−1 mg protein−1. The highest activity of CA was found in seeds hydroprimed in 24-epiBL (at 10−8 and 10−10 M, up to 0.28 and 0.24 nmol H2O2 min−1 mg protein−1, respectively, Fig. 4). The application of both SA and JA (at 10−3 M) also increased activity of CA to 0.25 and 0.24 nmol H2O2 min−1 mg protein−1, respectively.
Catalase activity in sunflower seeds after 21 days of chilling stress (0 °C). Seeds treatment was described on Fig. 1
Heat shock treatments applied together seed hydropriming in distilled water did not affect significantly the efficiency of PSII reaction centers in the dark-adapted state (F v/F m) in seedlings after recovery (Fig. 5). However, a slight positive tendency was observed comparing with non-treated and chilled control seedlings. Additionally, applied phytohormones together with heat shock during hydropriming significantly increased the maximum PSII efficiency (F v/F m) of dark-adapted leaves. The maximum quantum efficiency of photosystem II (PSII), as given by F v/F m, obtained 0.83 and 0.82 was influenced by 24-epiBL (at 10−8 and 10−10 M, respectively). The ratio of variable to maximal fluorescence (F v/F m) also significantly increased when SA or JA was applied at 10−3 M concentration.
Maximum PSII efficiency (F v/F m) of dark-adapted leaves of sunflower seedlings after 72 h of recovery from chilling stress. Seeds treatment was described on Fig. 1
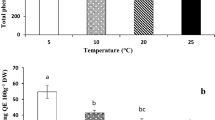
In cotyledons and growing tissues of seedling (roots with hypocotyl) after 72 h of recovery from the chilling stress, the same soluble carbohydrates occurred: monosaccharides (fructose and glucose), myo-inositol, sucrose, galactinol, raffinose and 1-kestose (Fig. 6). However, the concentration of total soluble carbohydrates was much higher in seedling tissues than that in cotyledons, regardless of seed treatment prior chilling stress. The concentration of glucose and fructose was ca. 100-fold higher (and remaining sugars twofold higher) in seedling than in cotyledons. Seeds conditioning in water had not significant (P < 0.05) effect on the concentrations of sugars in both seedling and cotyledons, as compared to seedlings developing from seeds without conditioning. The concentrations of glucose and sucrose in seedlings developing from seeds conditioned in solutions containing SA, JA and 24-epiBL was significantly (P < 0.05) higher than that in seedlings developed from control seeds and from seeds conditioned in water (Fig. 6a, c). Cotyledons from seeds treated with hormones contained higher amounts of sucrose (Fig. 6d). Moreover, JA and 24-epiBL reflected on increased concentrations of glucose, myo-inositol and raffinose (Fig. 6b, f, h). Conditioning had no significant effect on the concentration of 1-kestose in seedling and cotyledons tissues (Fig. 6g, h).
Soluble carbohydrates in seedlings (root with hypocotyl, a, c, e, g) and cotyledons (b, d, f, h). The concentration of fructose, glucose (a, b), sucrose (c, d), myo-inositol, galactinol (e, f), raffinose and 1-kestose (g, h) in seedlings and cotyledons after 72 h of recovery (at 25 °C) from chilling stress (21 days at 0 °C). Before chilling stress seeds were conditioned to 15% moisture content in water (H2O), solutions of salicylic (SA) or jasmonic acid (JA) at 10−3 M concentration and 24-epibrassinolide (BR) at 10−8M concentration and were heat shocked at 45 °C for 2 h. As a control (C) were used seeds without hydropriming (and non-heat shocked) and chilled at 0 °C for 21 days. The means of three replicates ±SE. Bars with the same letters are not significantly different (P < 0.05) after a Tukey’s correction for multiple comparisons
Discussion
Many plants are injured or killed by exposure to low non-freezing temperatures in the range of 0–15 °C. These species are classified as chilling-sensitive plants (Ishizaki-Nishizawa et al. 1996). Sunflower seedlings subjected to prolonged low temperatures exposure exhibited chilling injures manifested by inhibition of roots growth, longer mean emergence time and root damages expressed by external root discoloration as well as lower activities of dehydrogenases (Górnik et al. 2014). Hewezi et al. (2006) on the basis of an initial characterization of the transcriptome activity also found sunflower as a chilling-sensitive plant under suboptimal temperatures. Impairment of sunflower root growth at initial stages of plant development may result in poor crop establishment and higher susceptibility to pathogen attack (Groppa et al. 2008).
Therefore, methods which are able to reduce the chilling injuries are of great importance from both the theoretical and practical point of view. The obtained results shown that short-term heat shock applied during sunflower seed hydropriming in distilled water mitigated the adverse effect of chilling on radicles elongation (Fig. 1). Mangrich and Saltveit (2000) reported that chilling-sensitive okra, kenaf, cotton, and rice seedlings with radicles 10 mm long exposed to heat shock at 45 °C before being chilled, elongated more than seedlings not heat shocked before chilling. Our previous results have proved that heat shock applied earlier, before radicles protruded seed coat, may provide beneficial effects on chilling tolerance (Górnik et al. 2014). Hydropriming belongs to priming/conditioning techniques which uses only water in the process of controlled seed imbibitions (Grzesik and Nowak 1998). Posmyk et al. (2001) reported that osmoconditioning of soybean seeds reduced not only their chilling sensitivity but even allowed germination at 1 °C. In the present study, combining hydropriming and heat shock treatments revealed better effects on the acquisition of chilling tolerance by sunflower seedlings than the these two methods applied separately. The application of phytohormones during seed hydropriming together with short-term heat shock remarkably ameliorated inhibitory effects of chilling on roots elongation (Figs. 1, 2). Application of 24-epiBL was the most prominent in regards of roots growth protection against chilling conditions. The phytohormone has decreased detrimental effect of chilling to almost 20% compared to non-chilled control. Salicylic acid also positively affected sunflower roots elongation under low temperature suggesting that after that treatment seedlings conferred resistance to chilling conditions (Fig. 1). Janda et al. (1999) pointed out that maize plants, grown in hydroponic solutions, supplemented with 0.5 mM of SA attained resistance towards chilling or cold stress. The present study indicated also that application of JA during hydropriming protected root elongation against low temperature influence (Fig. 1). The observations are consistent with earlier findings indicating that methyl ester of JA, methyl jasmonate (MeJA), in priming solution improved watermelon seed germination at low temperatures which led to the higher percentages of seedling emergence (Korkmaz et al. 2004).
The present study clearly demonstrated that chilling suppressed the number of lateral roots development (i.e., 70% in comparison with non-chilled and non-heat shocked seedlings) (Fig. 2). Rab and Saltveit (1996) found in corn, cucumber, mung bean, and tomato that the number of lateral roots increased with increasing chilling duration up to 144 h, but then it suddenly declined as chilling duration continued to increase. Apparently, in our study, chilling period (3 weeks at 0 °C) for sunflower seeds exceeded the threshold of its inductive effect on the formation of lateral roots and suppresses lateral roots formation. Incorporation of phytohormones during seed hydropriming with heat shock application protected lateral root formation from negative chilling conditions. SA appeared to be the most effective in regards of lateral roots development, followed by 24-epiBL and JA (Figs. 2, 3). Wang et al. (2012) reported that JA might be one of the potent hormonal factors, other than auxin, involved in the regulation of lateral root formation.
Significant plant injures caused by low temperature stresses are related to oxidative damage at cellular level. Matsumura et al. (2002) reported that the increased chilling tolerance is associated with the enhanced activities of catalase (CA). This tetrameric enzyme effectively detoxifies H2O2 by its dismutating into water and oxygen. Bailly et al. (2004) stated that in mature sunflower seeds CA is mainly located in glyoxysomes. In the present study, its activity enhanced owing to seed hydropriming and heat shock treatment (Fig. 4). Prasad (1997) reported that CA plays one of the most important roles in acclimation to low temperature in maize seedlings by regulating the intracellular level of H2O2. In the present study, the highest activity of CA was observed in seeds hydroprimed in solution of 24-epiBL in concentration of 10−8 and 10−10 M, heat shocked for 2 h and returned to 25 °C (Fig. 4). According to Farooq et al. (2008), higher activity of CA under low temperature conditions causes a more efficient scavenging system which may result in better protection against ROS during stress. In our study, sunflower seed hydropriming combined with SA and JA also stimulated activity of CA (Fig. 4). Kang et al. (2003) also reported that during chilling stress period, SA pretreatment caused an activation of CA, which supports our results.
Low (non-freezing) stress also disrupts the activity of such photosynthetic apparatus like thylakoid electron transport, the carbon reduction cycle and control of stomatal conductance (Allen and Ort 2001). Therefore, the reduction of photosynthetic system injures caused by chilling is one of the most important challenges to research. In the present study, seed hydropriming with short-term heat shock treatment did not sufficiently reduced the decrease of the maximal photochemical efficiency of PSII (F v/F m) of dark-adapted leaves caused by chilling conditions (Fig. 4). However, inclusion of phytohormones during hydropriming revealed a significant enhancement of this parameter. The most pronounced results was obtained due to seeds treatment with 24-epiBL (at 10−8 and 10−10 M), suggesting that its application resulted in less dissipation of excitation energy in the PSII antennae and thereby reduced the chilling-induced photoinhibition. In other study, this phytohormone stimulated the intensity of the photosynthesis (Bajguz and Czerpak 1998) or prevents chilling injuries of photosynthetic apparatus by activating Calvin cycle enzymes and expression of photosynthetic genes. As a result, it led to the reduction of photo-oxidative stress and plant growth inhibition during recovery period after chilling (Xia et al. 2009). In the present study, application of SA or JA resulted also in increased the ratio of variable to maximal fluorescence (F v/F m) (Fig. 5). Apparently, the increased photosynthetic capacity after SA treatment was caused by its stimulatory effects on Rubisco activity, chlorophyll, various parameters of fluorescence as well as pigments content (Janda et al. 1998).
During seed germination and seedling development the composition and concentration of soluble carbohydrates changes according to the mobilization of storage materials degraded in embryonic axis, cotyledons and endosperm (Davies and Slack 1981). In our study, the major soluble carbohydrates were monosaccharides (glucose and fructose) and sucrose in seedling, and sucrose in cotyledons (Fig. 6). The severalfold higher concentration of monosaccharides in seedling than in cotyledons (Fig. 6a, b) confirms high metabolic activity of growing tissues and is characteristic for developing seedlings of sunflower (Pfeiffer and Kutschera 1996). In cotyledons of germinating sunflower seeds, glucose is synthesized by gluconeogenesis from the stored lipid breakdown (Pfeiffer and Kutschera 1996). The rate of this process was closely related to photosynthetic activity in cotyledons, and activity of enzymes engaged in sugars metabolism (Pfeiffer and Kutschera 1996). The low concentration of monosaccharides and severalfold higher concentration of sucrose in cotyledons, found in our study (Fig. 6b, d), was presumably a result of synthesis of sucrose which was transported into growing seedling. Borek et al. (2006) revealed that in germinating lupine seeds soluble sugars at the low level stimulate degradation of storage lipids by increasing lipase activity and β-oxidation. Authors concluded that the faster mobilization of storage materials in axes may be important for shaping the source–sink system in germinating lupine seeds. In Arabidopsis seeds, storage lipids are important but not essential for germination and seedling establishment (Kelly et al. 2011). Moreover, glucose at high concentration in growing seedling tissues can regulate several genes associated with seedling development (Rognoni et al. 2007). At the initial stages of seed germination and seedling development, both glucose and sucrose can be derived from other sources, than lipids or polysaccharides. In non-endospermic seeds, like legumes, during the initial stages of seed germination sucrose and raffinose family oligosaccharides (RFOs) play a role of primary sources of carbon skeletons and energy for tissues of growing seedling (Blöchl et al. 2007). The concentration of RFOs dramatically decreases in axis during the first 2 days of seed germination, and breakdown of RFOs is continued for next few days in cotyledons (Lahuta and Górecki 2011; Lahuta et al. 2014). The releasing sucrose is transported from cotyledons to the embryonic axis (seedling) for further metabolism (Kuo et al. 1988). The maturing seeds of sunflower accumulate raffinose (Lehner et al. 2006), which in turn is degraded during seeds germination (Kuo et al. 1988). Results of our study revealed that raffinose occurred in both seedling and cotyledons of sunflower, but at low level (below 0.5 and 1.0 mg g−1 DW, in cotyledons and seedling, respectively, Fig. 6g, h). It can be a result of incomplete raffinose breakdown during seed conditioning, chilling stress and recovery. However, the occurrence of galactinol, a galactosyl residues donor for synthesis of raffinose, in both seedling and cotyledons is more intriguing. It could be expected that during seed germination, galactinol should be fast degraded, as it is obvious in germinating legume seeds (Górecki et al. 2000). On the other hand, galactinol can be synthesized in vegetative tissues and transported in phloem of some species from source to sink tissues, or serve for synthesis of other phloem transported oligosaccharides—raffinose and stachyose. Alkio et al. (2002) indicated that raffinose is present in vegetative tissues of sunflower (stem and petiole) but is not phloem transported sugar. Another compound detected in seedling and cotyledons of sunflower was 1-kestose (Fig. 6g, h). The concentration of this fructan was low and similar to that of raffinose. This trisaccharide can be a product of fructans degradation and/or synthesis de novo in vegetative tissues (Martínez-Noël et al. 2015). Thus, the presence of galactinol, raffinose and 1-kestose in sunflower seedling seems to be explained. In regard to fact that galactinol, raffinose and fructans are accumulated in vegetative tissues of various plant species in response to several abiotic stresses (ElSayed et al. 2014), it can be speculated that the presence of above mentioned sugars in sunflower seedlings was an effect of tissues response to chilling stress. However, the confirmation of this hypothesis needs further studies.
The high concentrations of monosaccharides and sucrose in tissues increase cells osmotic potential necessary for water uptake and seedling elongation. In our study, the concentration of monosaccharides and sucrose was significantly higher in seedlings indicating faster growth rate (developing from seeds primed in SA, JA and 24-epiBL, Figs. 1, 2), than in the control. Therefore, it can be suggested that seeds priming in solutions containing SA, JA and 24-epiBL stimulates sugars metabolism. This stimulation can be associated with the regulation of metabolism leading to appropriate supply of substrates for synthesis of cellulose and hemicelluloses of primary cell walls in elongating seedlings (Sánchez-Rodríguez et al. 2010).
In our study, SA also increased concentration of sucrose and glucose in tissues of seedling and cotyledons (Fig. 6a–d). The increased concentration of sugars could function as osmotic regulators facilitating water uptake and retention in plant cells, thereby conferring seedlings development and additionally increasing tolerance to salinity stress (as in results of studies referred above) and/or chilling stress (as in our study).
While it seems very clear that jasmonates inhibit seed germination (Linkies and Leubner-Metzger 2012), it remains unclear how seed priming with jasmonate can stimulate development of seedling, as it was found in our study (Figs. 1, 2). In yellow lupine seeds, jasmonic acid-methyl ester (JA-ME) inhibited degradation of raffinose family oligosaccharides and α-d-galactosidase of cyclitols (presumably via inhibition of activity of α-d-galactosidase) and delayed seeds germination (Zalewski et al. 2010). Such inhibitory effect of JA-ME on seeds germination was obtained by application of JA-ME at 10−3 M concentration. However, the presence of JA at severalfold lower concentrations (10−6 and 10−9 M) during germination of Brassica napus seeds under NaCl stress stimulated accumulation of elevated amounts of soluble carbohydrates in 12-day old seedling (Kaur et al. 2013). Jasmonic acid affects changes in carbohydrate transport and partitioning (Babst et al. 2005) and can induce cell wall invertase in the phloem, regulating the content of sucrose (Millán-Cañongo et al. 2014). Thus, it seems to be possible that the presumably elevated level of JA in primed sunflower seeds (in our study) can stimulate changes in soluble sugars translocation between cotyledons and root during seedling development.
The presented data indicated that hydropriming and short-term heat shock application explicitly reduced inhibition of roots as well as lateral roots development by allowing the germinating seeds to recover from the growth-inhibiting effects of chilling. Seeds hydropriming in solutions containing SA, JA and 24-epiBL followed heat shock treatment additionally promoted the activity of catalase and sugars metabolism, which stimulated seedlings development and alleviated the decrease of F v/F m caused by chilling conditions. These beneficial effects lead to increased resistance of sunflower seedlings to chilling stress. The present study demonstrated that short-term heat shock applied during hydropriming and application of 24-epiBL, salicylic and jasmonic acid can be used as an effective method to improve sunflower (H. annuus L.) establishment at low temperature.
The present study demonstrated that the most profitable effect on reducing negative effect of chilling on inhibition of radicle growth, the inhibition of the number of lateral roots formation, the activity of catalase and changes in soluble carbohydrates in seedlings may be achieve by short-term heat shock applied during hydropriming in water supplemented with 24-epiBL (10−8 and 10−10 M) and salicylic acid (10−3 and 10−4 M). Jasmonic acid enhanced the examined parameter to a lesser extent than 24-epiBL or salicylic acid.
Author contribution statement
Krzysztof Górnik prepared experimental design, carried out most of the research and was the main author of this manuscript. Lesław B. Lahuta conducted experiments concerning soluble carbohydrate analysis, helped with manuscript preparation, proposed conception of the study concerning soluble carbohydrate analysis, discussed the results and commented on the manuscript.
Abbreviations
- 24-epiBL:
-
24-Epibrassinolide
- SA:
-
Salicylic acid
- MeSA:
-
Methyl salicylate
- JA:
-
Jasmonic acid
- MeJA:
-
Methyl jasmonate
- CAT:
-
Catalase
References
Alkio M, Diepenbrock W, Grimm E (2002) Evidence for sectorial photoassimilate supply in the capitulum of sunflower (Helianthus annuus). New Phytologist 156:445–456
Babst BA, Ferrieri RA, Gray DW, Lerdau M, Schlyer DJ, Schueller M, Thorpe MR, Orians CM (2005) Jasmonic acid induces rapid changes in carbon transport and partitioning in Populus. New Phytol 167(1):63–72
Bailly C, Benamar A, Corbineau F, Come D (1996) Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Physiol Plant 97(1):104–110
Bailly C, Leymarie J, Lehner A, Rousseau S, Côme D, Corbineau F (2004) Catalase activity and expression in developing sunflower seeds as related to drying. J Exp Bot 55(396):475–483
Bajguz A, Czerpak R (1998) Physiological and biochemical role of brassinosteroids and their structure-activity relationship in the green alga Chlorella vulgaris Beijerinck (Chlorophyceae). J Plant Growth Regul 17(3):131–139
Balasaraswathi R, Sadasivam S (1997) Changes in oil, sugars and nitrogenous components during germination of sunflower seeds, Helianthus annuus. Plant Foods Hum Nutr 51:71–77
Blöchl A, Peterbauer T, Richter A (2007) Inhibition of raffinose oligosaccharide breakdown delays germination of pea seeds. J Plant Physiol 164(8):1093–1096
Borek S, Ratajczak W, Ratajczak L (2006) Ultrastructural and enzymatic research on the role of sucrose in mobilization of storage lipids in germinating yellow lupine seeds. Plant Sci 170(3):441–452
Davies HV, Slack PT (1981) The control of food mobilization in seeds of dicotyledonous plants. New Phytol 88(1):41–51
ElSayed AI, Rafudeen MS, Golldack D (2014) Physiological aspects of raffinose family oligosaccharides in plants: protection against abiotic stress. Plant Biol 16(1):1–8
Fahad S, Hussain S, Bano A, Saud S, Hassan S, Shan D, Khan FA, Khan F, Chen Y, Wu C (2014) Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: consequences for changing environment. Environ Sci Pollut Res 22(7):4907–4921
FAOSTAT (2014) Food and Agriculture Organization of The United Nations Statistics Division. http://www.fao.org/faostat/en/#data/QC. Accessed 2014
Farooq M, Aziz T, Basra SM, Cheema MA, Rehman H (2008) Chilling tolerance in hybrid maize induced by seed priming with salicylic acid. J Agron Crop Sci 194(2):161–168
Górecki RJ, Fordonski G, Halmajan H, Horbowicz M, Jones RG, Lahuta LB, Hedley CL (2000) Seed physiology and biochemistry. In: Hedley CL (ed) Carbohydrates in grain legume seeds: improving nutritional quality and agronomic characteristics. CAB International, Wallingford, pp 117–143
Górnik K, Badowiec A, Weidner S (2014) The effect of seed conditioning, short-term heat shock and salicylic, jasmonic acid or brasinolide on sunflower (Helianthus annuus L.) chilling resistance and polysome formation. Acta Physiol Plant 36(10):2547–2554
Groppa MD, Zawoznik MS, Tomaro ML, Benavides MP (2008) Inhibition of root growth and polyamine metabolism in sunflower (Helianthus annuus) seedlings under cadmium and copper stress. Biol Trace Elem Res 126:246–256. doi:10.1007/s12011-008-8191-y
Grzesik M, Nowak J (1998) Effects of matriconditioning and hydropriming on Helichrysum bracteatum L. seed germination, seedling emergence and stress tolerance. Seed Sci Technol 26(2):363–376
Hewezi T, Léger M, Kayal WE, Gentzbittel L (2006) Transcriptional profiling of sunflower plants growing under low temperatures reveals an extensive down-regulation of gene expression associated with chilling sensitivity. J Exp Bot 57(12):3109–3122. doi:10.1093/jxb/erl080
Ishizaki-Nishizawa O, Fujii T, Azuma M, Sekiguchi K, Murata N, Ohtani T, Toguri T (1996) Low-temperature resistance of higher plants is significantly enhanced by a nonspecific cyanobacterial desaturate. Nat Biotechnol 14:1003–1006
Janda T, Szalai G, Antunovics ZS, Ducruet JM, Paldi E (1998) Effects of salicylic acid and related compounds on photosynthetic parameters in young maize (Zea mays L.) plants. Photosynthesis: mechanisms and effects. Kluwer Academic Publishers, Dordrecht, pp 3869–3872
Janda T, Szalai G, Tari I, Paldi E (1999) Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta 208(2):175–180
Janda T, Gondor OK, Yordanova R, Szalai G, Pál M (2014) Salicylic acid and photosynthesis: signalling and effects. Acta Physiol Plant 36(10):2537–2546
Kang H-M, Saltveit ME (2001) Activity of enzymatic antioxidant defense systems in chilled and heat shocked cucumber seedling radicles. Physiol Plant 113(4):548–556. doi:10.1034/j.1399-3054.2001.1130414.x
Kang G, Wang C, Sun G, Wang Z (2003) Salicylic acid changes activities of H2O2-metabolizing enzymes and increases the chilling tolerance of banana seedlings. Environ Exp Bot 50(1):9–15
Kaur H, Sharma P, Sirhindi G (2013) Sugar accumulation and its regulation by jasmonic acid in Brassica napus L. under salt stress. J Stress Physiol Biochem 9(4):53–64
Kelly AA, Quettier A-L, Shaw E, Eastmond PJ (2011) Seed storage oil mobilization is important but not essential for germination or seedling establishment in Arabidopsis. Plant Physiol 157(2):866–875
Korkmaz A, Tiryaki I, Nas MN, Ozbay N (2004) Inclusion of plant growth regulators into priming solution improves low-temperature germination and emergence of watermelon seeds. Can J Plant Sci 84(4):1161–1165. doi:10.4141/P04-028
Kuo TM, VanMiddlesworth JF, Wolf WJ (1988) Content of raffinose oligosaccharides and sucrose in various plant seeds. J Agric Food Chem 36(1):32–36
Lahuta LB (2006) Biosynthesis of raffinose family oligosaccharides and galactosyl pinitols in developing and maturing seeds of winter vetch [Vicia villosa Roth.]. Acta Soc Bot Polon 75(3):219–227
Lahuta LB, Górecki RJ (2011) Raffinose in seedlings of winter vetch (Vicia villosa Roth.) under osmotic stress and followed by recovery. Acta Physiol Plant 33(3):725–733. doi:10.1007/s11738-010-0597-4
Lahuta LB, Pluskota WE, Stelmaszewska J, Szablińska J (2014) Dehydration induces expression of galactinol synthase and raffinose synthase in seedlings of pea (Pisum sativum L.). J Plant Physiol 171(14):1306–1314
Lehner A, Corbineau F, Bailly C (2006) Changes in lipid status and glass properties in cotyledons of developing sunflower seeds. Plant Cell Physiol 47(7):818–828
Linkies A, Leubner-Metzger G (2012) Beyond gibberellins and abscisic acid: how ethylene and jasmonates control seed germination. Plant Cell Rep 31(2):253–270
Mangrich ME, Saltveit ME (2000) Heat shocks reduce chilling sensitivity of cotton, kenaf, okra, and rice seedling radicles. J Am Soc Hortic Sci 125(3):377–382
Martínez-Noël GMA, Dosio GAA, Puebla AF, Insani EM, Tognetti JA (2015) Sunflower: a potential fructan-bearing crop? Front Plant Sci. doi:10.3389/fpls.2015.00798
Matsumura T, Tabayashi N, Kamagata Y, Souma C, Saruyama H (2002) Wheat catalase expressed in transgenic rice can improve tolerance against low temperature stress. Physiol Plant 116(3):317–327
Millán-Cañongo C, Orona-Tamayo D, Heil M (2014) Phloem sugar flux and jasmonic acid-responsive cell wall invertase control extrafloral nectar secretion in Ricinus communis. J Chem Ecol 40(7):760–769
Pál M, Gondor O, Janda T (2013) Role of salicylic acid in acclimation to low temperature. Acta Agron Hung 61(2):161–172
Pfeiffer I, Kutschera U (1996) Sucrose metabolism and lipid mobilization during light-induced expansion of sunflower cotyledons. J Plant Physiol 147(5):553–558
Posmyk MM, Corbineau F, Vinel D, Bailly C, Côme D (2001) Osmoconditioning reduces physiological and biochemical damage induced by chilling in soybean seeds. Physiol Plant 111(4):473–482
Prasad TK (1997) Role of catalase in inducing chilling tolerance in pre-emergent maize seedlings. Plant Physiol 114(4):1369–1376
Rab A, Saltveit ME (1996) Sensitivity of seedling radicles to chilling and heat-shock-induced chilling tolerance. J Am Soc Hortic Sci 121(4):711–715
Rognoni S, Teng S, Arru L, Smeekens SCM, Perata P (2007) Sugar effects on early seedling development in Arabidopsis. Plant Growth Regul 52(3):217–228
Sánchez-Rodríguez C, Rubio-Somoza I, Sibout R, Persson S (2010) Phytohormones and the cell wall in Arabidopsis during seedling growth. Trends Plant Sci 15(5):291–301
Wang Y, Hu J, Qin G, Cui H, Wang Q (2012) Salicylic acid analogues with biological activity may induce chilling tolerance of maize (Zea mays) seeds. Botany 90(9):845–855
Xia X-J, Huang L-F, Zhou Y-H, Mao W-H, Shi K, Wu J-X, Asami T, Chen Z, Yu J-Q (2009) Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 230(6):1185–1196
Zalewski K, Nitkiewicz B, Lahuta LB, Głowacka K, Socha A, Amarowicz R (2010) Effect of jasmonic acid–methyl ester on the composition of carbohydrates and germination of yellow lupine (Lupinus luteus L.) seeds. J Plant Physiol 167(12):967–973
Zhang YH, Chen LJ, He JL, Qian LS, Wu LQ, Wang RF (2010) Characteristics of chlorophyll fluorescence and antioxidative system in super-hybrid rice and its parental cultivars under chilling stress. Biol Plant 54(1):164–168
Zhang Z, Zhu Q, Hu M, Gao Z, An F, Li M, Jiang Y (2017) Low-temperature conditioning induces chilling tolerance in stored mango fruit. Food Chem 219:76–84
Zhou B, Guo Z (2009) Calcium is involved in the abscisic acid-induced ascorbate peroxidase, superoxide dismutase and chilling resistance in Stylosanthes guianensis. Biol Plant 53(1):63–68
Acknowledgements
This work was supported by Grant NoN310 089436 founded by the Ministry of Science and Higher Education, Poland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O Ferrarese-Filho.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Górnik, K., Lahuta, L.B. Application of phytohormones during seed hydropriming and heat shock treatment on sunflower (Helianthus annuus L.) chilling resistance and changes in soluble carbohydrates. Acta Physiol Plant 39, 118 (2017). https://doi.org/10.1007/s11738-017-2413-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-017-2413-x