Abstract
The role of sodium nitroprusside (SNP, 10 μM), a nitric oxide (NO) donor, and/or methyl jasmonate (MJ, 100 μM) spiked with l-phenylalanine (PHEN, 100 μM) and additional sucrose (S; 30 g l−1), in taxane production and phenyl ammonia lyase (PAL) activity in cultures of two Taxus media x var. Hicksii transgenic root lines (ATMA and ATM) carrying the taxadiene synthase transgene was investigated. SNP addition, when applied together with MJ and/or PHEN, resulted in paclitaxel production only in ATMA cultures. The application of the NO donor gave the highest paclitaxel content (7.56 mg l−1) in the combination of SNP+S+MJ+PHEN, after 2 weeks of treatment in the ATMA root line. In ATM cultures, taxane production was not affected by SNP. In both ATMA and ATM lines the highest total (intra+extracellular) paclitaxel yield was determined when elicited with MJ+PHEN, and amounted to 10.78 mg l−1 at 1 week and 1.63 mg l−1 at 2 weeks of treatment, in cultures of ATMA and ATM lines, respectively. The excretion of paclitaxel was observed only in ATMA cultures, with the highest level (2.34 mg l−1) obtained after elicitation with S+MJ+PHEN. The comparison of PAL activity in the two root lines revealed that this enzyme was almost 3-times more active in ATM than ATMA roots. An increase in both PAL activity and paclitaxel production was only observed in ATMA cultures growing in medium supplemented with S+MJ+PHEN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paclitaxel is the active agent of Taxol® (Bristol-Myers Squibb), one of the most effective anticancer plant-derived drugs. Paclitaxel is not only a first line treatment chosen for ovary, breast and lung cancers, but is also used in combination therapies (Fauzee 2011). Moreover, paclitaxel has shown great promise as a locally delivered antirestenotic agent and also seems to be an effective compound for the treatment of Alzheimer’s and other neurodegenerative diseases (Nims et al. 2006; Li et al. 2003; Fu et al. 2009).
A very promising approach for the production of paclitaxel and related taxanes, without forest harvesting, is provided by plant biotechnology approaches, and taxane production has been recently successfully scaled up (Frense 2007). Many strategies leading to the enhancement of taxane accumulation in Taxus in vitro cultures have been investigated (Zhong 2002; Tabata 2004, 2006; Exposito et al. 2010; Onrubia et al. 2013; Sabater-Jara et al. 2014). Among them, medium supplementation with elicitor/s and/or precursors has proved to be the most effective.
Nitric oxide (NO), a gaseous free radical, is involved in numerous physiological processes in plants, including plant responses to biotic and abiotic stress (Wojtaszek 2000; Lamotte et al. 2005; Palavan-Unsal and Arisan 2009; Misra et al. 2010; Scheler et al. 2013; Trapet et al. 2014). Indications that NO is involved in signaling defense responses during plant–pathogen interactions are well documented (Sudha and Ravishankar 2002; Arasimowicz and Floryszak-Wieczorek 2007; Zhang et al. 2012; Scheler et al. 2013; Yu et al. 2014; Trapet et al. 2014). Induced NO generation has also been reported, using various fungal elicitors, which mediate the accumulation of secondary metabolites belonging to diverse chemical groups (Zhang et al. 2012).
The signaling role of NO in the enhancement of both paclitaxel production and PAL activity in Taxus sp. in vitro cultures has been confirmed (Wang and Wu 2004, 2005; Xu et al. 2004; Wang et al. 2006; Xiao et al. 2009). Although PAL does not participate in the formation of the paclitaxel C-13 side chain, several inhibitors of this enzyme were found to stop paclitaxel production almost entirely (Brincat et al. 2002).
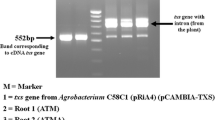
The first committed step in the synthesis of paclitaxel and its congeners is the cyclization of geranylgeranyl pyrophosphate (GGPP) by taxadiene synthase (txs synthase) (Croteau et al. 2006). Although this enzyme does not seem to be rate-limiting in taxane production, the presence of both the txs synthase transgene and rol genes of Agrobacterium rhizogenes in Taxus x media cell suspension cultures has led to significantly higher levels of taxanes in comparison with cell lines transformed only by A. rhizogenes wild strain (Exposito et al. 2010). Moreover, the txs synthase transgene has recently been successfully introduced in Taxus x media var. Hicksii, resulting in taxaneproducing transgenic hairy root cultures (Sykłowska-Baranek et al. 2015).
In this study, we compared the effect of two signaling molecules, NO and MJ, spiked or not with l-phenylalanine (PHEN) (a precursor in the taxane biosynthesis pathway) on taxane production in two lines of Taxus x media var. Hicksii hairy roots carrying a taxadiene synthase transgene (txs gene). Simultaneously, the activity of PAL, the key enzyme in phenylpropanoid compound biosynthesis, was determined.
Although there have been some papers reporting the effect of MJ and NO elicitation on paclitaxel production in cell suspension cultures of Taxus spp. there is no publications treating differentiated organs and especially transformed roots. Hairy roots, representing differentiated cell system which is believed to differ in response to elicitors in comparison to undifferentiated cells, moreover due to the fact that Taxus plants are invulnerable to genetic transformation, the investigation of their potential to taxane production is scarce. To our knowledge, this is the first report on the elucidation of the signal role of NO in elicitor-induced PAL activation and taxane production in Taxus x media var. Hicksii hairy roots and carrying the txs transgene.
Materials and methods
Hairy root cultures
Taxus x media var. Hicksii hairy root cultures were established as described earlier by Sykłowska-Baranek et al. (2015) by transformation of 10-year-old Taxus plantlets cultivated in vitro with C58C1 strain of Agrobacterium tumefaciens carrying the plasmid RiA4 of A. rhizogenes and the binary plasmid pCAMBIA-TXS-His, harboring the taxadiene synthase (txs) gene of T. baccata (GenBank accession: AY424738), under the control of the 35S CaMV promoter, and the hygromycin phosphotransferase gene (hptII) as a resistance marker. Transgenic roots were routinely cultured in 250 ml Erlenmeyer flasks with 35 ml hormone-free DCR-M medium (Syklowska-Baranek et al. 2009) with a subculture in fresh medium every 4 weeks. The cultures were maintained at 25 ± 1 °C in the dark on an INFORS AG TR 250 shaker (Switzerland) at 105 rpm.
Elicitor and precursor feeding experiments
The nitric oxide donor—sodium nitroprusside (SNP; 10 μM, Sigma-Aldrich) was added to the medium alone or in combination with methyl jasmonate (MJ; 100 μM, Sigma-Aldrich) and/or l-phenylalanine (PHE; 100 μM, Sigma-Aldrich) and sucrose (S; 30 g l−1, POCH, Poland).
The solution of PHE and sucrose was autoclaved prior to use. SNP was dissolved in distilled water and filtered through Sartorius 0.20 μm PTFE filter prior to application. MJ was dissolved in EtOH (POCH, Poland) aseptically and applied to the cultures after autoclaving.
In all experiments each flask was inoculated with 0.36 ± 0.025 g fresh weight (FW) of transgenic roots. At day 28 of culture, hairy roots growing in Erlenmeyer flasks with 35 ml DCR-M were supplemented with elicitors and/or PHE and additional sucrose. The cultures were maintained for two more weeks with samples harvested after 24, 48 h and 1 and 2 weeks. At each time point, the roots from three flasks were harvested, gently pressed on paper, and their fresh weight was recorded. After lyophilisation, the dry weight (DW) of roots was determined. The fresh biomass increase was expressed as the ratio of final weight to initial weight.
The concentration of MJ and PHE, as well as the period of exposure to their treatment was chosen on the basis of our earlier investigations (Syklowska-Baranek et al. 2009) while the SNP dosage was inspired by the results obtained by Wang and Wu (2005).
Taxane determination
The content of six taxanes, paclitaxel, taxol C, 10-deacetyltaxol, 7-epi-10-deacetyltaxol, baccatin III and 10-deacetylbaccatin III, was determined in powdered dry tissue of transgenic roots, as well as in medium. The samples were cleaned using the method described by Theodoridis et al. (1998a). Prior to HPLC analysis, samples were re-dissolved in 100 % MeOH (400 μl) and 20 μl underwent HPLC–DAD analysis using the DIONEX (USA) system with a UVD 340S diode array detector and an automated sample injector (ASI-100). For compound separation, a Kinetex (Phenomenex, USA) 100 × 4.60 mm column was eluted employing the gradient program, as described by Theodoridis et al. (1998b). The DAD spectrophotometer was set at a wavelength range of 215–275 nm. The taxanes were identified and quantified at 227 nm. The peaks were assigned by spiking the samples with the standards and comparing the retention times and UV spectra.
The standard compounds were produced by CHOMADEX (USA) and purchased from LCG Standards (Poland). All chemicals were of HPLC-grade and purchased from Sigma-Aldrich.
All experiments were performed in triplicate. The statistical significance between means was assessed using analysis of variance (ANOVA) and Tukey’s multiple range test. A probability of p = 0.05 was considered significant.
PAL activity assays
PAL activity was measured according to the method of Zucker (1965), with the modifications (Sykłowska-Baranek et al. 2012). The protein content was determined using the method of Bradford (1976).
All reagents were purchased from Sigma-Aldrich.
Results and discussion
Characterization of hairy root growth
The growth of two transgenic root lines carrying the txs transgene was investigated, comparing control cultures (without any additives) with cultures supplemented at day 28 with a precursor (PHE), additional sucrose (S) and elicitor/s MJ and/or SNP.
In control cultures of ATMA and ATM roots, the highest fresh biomass increase was noted at the end of the experiment at day 42. At that time, the ATM roots achieved significantly higher (p = 0.05) biomass accumulation than ATMA roots, 1.78-fold ± 0.2 and 1.90-fold ± 0.18, respectively. Stimulation of root growth, in relation to the control, was observed in cultures elicited solely with SNP (Fig. 1a, b), without a significant difference (p = 0.05) between the two root lines. Under these conditions, the highest biomass increase was determined at the end of the culture at day 42, being 2.07-fold ± 0.35 and 2.30-fold ± 0.15 in ATMA and ATM cultures, respectively.
Fresh biomass increase (expressed as ratio of final weight to initial weight) of Taxus x media var. Hicksii transgenic roots carrying the taxadiene synthase transgene, cultured in various modifications of DCR-M medium: a ATMA root line; b ATM root line. Data represent mean value of 3 replicates ± SD (standard deviation)
In media containing SNP together with other additives, root growth decreased, especially in ATM cultures (Fig. 1a, b), with the lowest growth index (0.78-fold ± 0.19) observed in SNP+S medium. At the same time, it was the lowest value of biomass increase obtained for this root line. In ATMA root cultures the most pronounced growth inhibition was caused, not by SNP alone or in combination with other elicitors and/or precursors, but by medium supplementation with MJ + PHEN (0.79 ± 0.16), MJ (0.93 ± 0.05) and S+MJ (0.75 ± 0.14).
In a previous investigation on ATMA hairy root cultures, we observed a similar inhibitory effect of MJ treatment on root growth (Sykłowska-Baranek et al. 2015), with a 32 % growth reduction in relation to the control. MJ-induced growth inhibition, irrespective of the presence of precursors in the medium, was also noted in wild type (transformation carried out with LBA 9402 A. rhizogenes strain) hairy root cultures of T. x media var. Hicksii (Furmanowa and Syklowska-Baranek 2000). Exposito et al. (2010), followed by Onrubia et al. (2012), also noted a cell growth reduction of about 30 % in MJ-elicited (100 μM T. media) cell suspension cultures harboring the txs gene in comparison with an unelicited control. The detrimental effect of MJ on root and cell growth could be attributed to its induction of H2O2 production (Wang and Wu 2005). In T. chinensis cell suspension cultures, SNP alone did not cause H2O2 formation and was responsible for only 7.1 % of cell death (Wang et al. 2006). Moreover, supplementing the medium with SNP suppressed the MJ-induced production of H2O2 (Wang and Wu 2005).
Taxane production and PAL activity
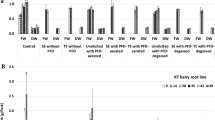
No taxanes were detected in the two root lines carrying the txs transgene cultivated in control conditions and without MJ. The presence of MJ in the medium was necessary to induce paclitaxel accumulation, as this taxane was the only one determined both in dry biomass and post-culture media (Fig. 2a, b).
Total (intra + extracellular) paclitaxel content (mg l−1) in Taxus x media var. Hicksii transgenic roots carrying the taxadiene synthase transgene, cultured in various modifications of DCR-M medium: a ATMA root line; b ATM root line. Data represent mean value of 3 replicates ± SD (standard deviation)
Line ATMA was characterized by a higher capacity for paclitaxel production than ATM. The total (intra+extracellular) paclitaxel content in ATMA roots was highest (10.78 ± 1.50 mg l−1) after 1 week of elicitation with MJ + PHEN, being almost 7-times more than the highest amount in ATM roots (1.63 ± 0.89 mg l−1) cultivated under the same conditions but elicited for 2 weeks (Fig. 2b).
In the same conditions (2 weeks of elicitation in MJ+PHEN media), ATMA roots accumulated in dry biomass 3-fold higher amounts of intracellular paclitaxel than ATM roots. Extracellular paclitaxel was also found, but only in ATMA roots, with the highest level (2.34 ± 0.37 mg l−1) obtained under S+MJ+PHE treatment for 1 week.
SNP addition resulted in paclitaxel production only in combination with MJ, alone or with PHEN (Fig. 2). The total paclitaxel detected in ATMA roots cultured in SNP+S+MJ media amounted to 2.74 ± 1.35 and 3.79 ± 1.19 mg l−1 after 1 and 2 weeks of elicitation, respectively. The application of PHEN together with SNP+S+MJ significantly enhanced the total paclitaxel yield (7.56 ± 2.27 mg l−1 at the end of culture) and excretion of paclitaxel to the medium was also observed (0.04 ± 0.01 mg l−1). ATM roots treated with SNP+S+MJ were able to accumulate paclitaxel only for 48 h after elicitation, with an output of 0.07 ± 0.01 mg l−1 (Fig. 2b).
The pattern of PAL activity differed substantially between the two transgenic root lines (Fig. 3). Comparing the two root lines revealed that PAL was almost 3-times more active in ATM than ATMA roots (Fig. 3a, b). In ATMA roots PAL activity was observed to peak after 1 week both in control and elicited cultures, except under SNP, MJ and MJ+PHEN treatments (Fig. 3a). Moreover, the lowest PAL levels were noted in MJ or MJ+PHEN media, while the highest paclitaxel yield was achieved with the addition of MJ+PHEN (Figs. 2a, 3a). The maximum activity of PAL in the ATMA line was detected under S+MJ+PHEN treatment and simultaneously correlated with the highest paclitaxel accumulation in this variant of culture.
A dramatic increase in PAL activity was observed in ATM cultures 24 h after elicitation, with or without the precursor (Fig. 3b), with the highest values determined in roots treated with S+MJ, SNP+S+MJ+PHEN, S+MJ+PHEN, SNP+S+MJ and SNP+S, in diminishing order. A second rise in PAL activity, distinct but significantly lower than the first, took place after 1 week of elicitation. No paclitaxel was detected at the day of maximum PAL levels, nor was it produced in ATM cultures maintained in the SNP+S+MJ+PHEN medium during the 2 weeks of elicitation. In cultures of ATM roots growing for 24 h in the presence of MJ or MJ+PHEN, PAL activity was on the same level as in the control, but paclitaxel had already been produced (Figs. 2, 3b). Moreover, the highest paclitaxel content in ATM roots was detected when the PAL level was almost the lowest.
The presence of cross-talk in complex, cellular signaling networks between various signaling molecules like MJ and NO, mediating a large number of biological processes in plants, including responses to various stress conditions, has been reported (see review by Qiao et al. 2014; Wasternack 2014; Dar et al. 2015). Wang and Wu (2005), on the basis of results obtained in T. chinensis suspension cultures, proposed the transduction signal pathway starting from MJ through NO with subsequent PAL activation leading to enhanced paclitaxel accumulation.
Earlier reports have also indicated the substantial influence of NO on the accumulation of paclitaxel and other taxanes in Taxus sp. in vitro cultures. Xiao et al. (2009) investigated the production and distribution of NO in immobilized suspension cultures of T. cuspidata. The highest paclitaxel content coincided with the highest NO accumulation in cell aggregates. Moreover, the application of SNP as an NO donor resulted in an 11 % rise in paclitaxel yield.
The generation of NO and its regulatory role in paclitaxel production and PAL activation was also confirmed in T. yunnanensis cells treated with fungal-derived cerebroside by Wang et al. (2007a) and in T. chinensis cells treated with a fungal elicitor (Wang and Wu 2004). In suspension cultures of T. yunnanensis the generation of NO by low-energy ultrasound (US) treatment or application of SNP significantly stimulated PAL activity (Wang et al. 2006). Although, PAL activity was higher under simultaneous US and SNP treatment, which indicated a positive effect of NO on this enzyme, the SNP alone or in combination with US caused a slight improvement in paclitaxel content. US treatment resulted in a distinct increase in paclitaxel (15-fold) and baccatin III (8-fold) compared to the control.
Moreover, the correlation in increase of PAL activity combined with enhanced paclitaxel content has been reported in Taxus in vitro cultures treated with various elicitors. In suspension cultures of T. yunnanensis, Zhang et al. (2002) described a peak in PAL activity 24 h after elicitation with a mixture of MJ, Ag+ and chitosan, which lasted for more than 8 days. The PAL activity depended mainly on the inoculum age, and higher enzyme levels corresponded with a higher paclitaxel yield. PAL activity in suspension cultures of T. chinensis was stimulated by MJ, peaking at 15 days after treatment (Wu and Lin 2003), and paclitaxel accumulation was also enhanced. In chitosan-adapted suspension cultures of T. chinensis, MJ application increased paclitaxel production and a 10-fold higher peak of PAL activity was noted after 1.5 days (Zhang et al. 2007). Similar results were observed by Wang et al. (2007b) in T. chinensis var. mairei under the influence of salicylic acid.
In our experiments, SNP alone neither stimulated PAL activity nor taxane production. But SNP applied together with MJ or with MJ and PHEN exhibited a synergetic effect on paclitaxel accumulation and PAL activity, which is in accordance with the signal transduction pathway proposed by Wang and Wu (2005). However, SNP was not indispensable for the highest paclitaxel productivity and PAL activity (Figs. 2, 3). These results suggest the dominance of the jasmonate-mediate signal transduction pathway in paclitaxel biosynthesis.
The highest paclitaxel content was determined in ATMA and ATM root lines cultivated in media supplemented with PHEN, which is not surprising since PHEN provides both the phenylisoserine side chain and the C-2 benzoyl moiety of paclitaxel (Brincat et al. 2002).
The discrepancy in PAL activity observed between investigated root lines might be attributed to the significantly higher accumulation of trans-cinnamic acid in the ATM line within 24H and its inhibitory impact on PAL activity, resulting in a substantially lower paclitaxel productivity in comparison with the ATMA line. A persistent reduction in paclitaxel yield under trans-cinnamic acid treatment was also reported by Brincat et al. (2002). In the ATMA line, the peak in PAL activity was detected after 1 week of elicitation, which corresponded with the highest paclitaxel levels in media elicited with MJ but without SNP (Figs. 2, 3). In cultures elicited with SNP and MJ, the highest paclitaxel content was detected after 2 weeks of elicitation. These results support the findings by Brincat et al. (2002), who related the impact of trans-cinnamic acid on paclitaxel production not only with its direct effect on PAL but also on other steps of the taxane biosynthetic pathway, e.g. those involving the lipoxygenase enzyme (LOX). LOX activity has been positively correlated with paclitaxel accumulation in elicited T. chinensis cells (Huang et al. 2005; Wang and Wu 2005). Moreover, Li et al. (2012) observed strong up-regulation of the TcLOX1 LOX gene, cloned from T. chinensis cells and preferentially expressed in stems and roots, in response to MJ elicitation.
The results described here confirm that the paclitaxel yield obtained by biotechnological methods is influenced by many factors, including the culture type and its state of development and differentiation, the kind of elicitor and/or precursor, and their concentration and duration of treatment.
Conclusions
The effect of different elicitors (MJ, SNP as an NO donor, and both together) with or without PHEN, and additional sugar on taxane content and PAL activity in two transgenic T. x media var. Hicksii root lines carrying the txs transgene was studied. Out of six searched taxanes, only paclitaxel was detected. The two examined root lines differed significantly in their capacity for paclitaxel production, which was not induced by SNP alone. The target compound was only achieved in media containing MJ, with its levels being higher with the additional presence of the precursor PHEN. PAL activity coincided with paclitaxel production in the ATMA line cultured in media supplemented with MJ alone or S+MJ+PHEN. However, the highest enzyme activity was determined in ATM cultures, where it was not accompanied by an enhancement in paclitaxel yield.
Due to the high commercial value of paclitaxel and other taxanes, it seems reasonable to undertake further efforts to elucidate and manipulate the metabolic flux to obtain a more efficient production.
Author contribution statement
Katarzyna Sykłowska-Baranek planned the investigations, carried out transgenic root cultures, performed HPLC UV-DAD analysis, elaborated the results and prepared the manuscript. Marta Grech-Baran carried out transgenic root cultures, elaborated results and participated in manuscript preparation. Mercedes Bonfill prepared modified Agrobacterium tumefaciens strain with txs gene insert and carried out PCR analysis, elaborated the results and participated in manuscript preparation. Marcin Robert Naliwajski performed PAL activity determination and participated in manuscript preparation. Agnieszka Pietrosiuk elaborated statistically results and participated in manuscript preparation.
References
Arasimowicz M, Floryszak-Wieczorek J (2007) Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant Sci 172:876–887. doi:10.1016/j.plantsci.2007.02.005
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Brincat MC, Gibson DM, Shuler ML (2002) Alterations in Taxol production in plant cell culture via manipulation of the phenylalanine ammonia lyase pathway. Biotechnol Prog 18:1149–1156. doi:10.1021/bp0256115
Croteau R, Ketchum REB, Long RM, Kaspera R, Wildung MR (2006) Taxol biosynthesis and molecular genetics. Phytochem Rev 5:75–97. doi:10.1007/s11101-005-3748-2
Dar TA, Uddin M, Khan MMA, Hakeem KR, Jaleel H (2015) Jasmonates counter plant stress: a review. Environ Exp Bot 115:49–57. doi:10.1016/j.envexpbot.2015.02.010
Exposito O, Syklowska-Baranek K, Moyano E, Onrubia M, Bonfill M, Palazon J, Cusido RM (2010) Metabolic responses of Taxus media transformed cell cultures to the addition of methyl jasmonate. Biotechnol Prog 26:1145–1153. doi:10.1002/btpr.424
Fauzee NJS (2011) Taxanes: promising anti-cancer drugs. Asian Pac J Cancer Prev 12:837–851
Frense D (2007) Taxanes: perspectives for biotechnological production. Appl Microbiol Biotech 73:1233–1240. doi:10.1007/s00253-006-0711-0
Fu Y, Li S, Zu Y, Yang G, Yang Z, Luo M, Jiang S, Wink M, Efferth T (2009) Medicinal chemistry of paclitaxel and its analogues. Curr Med Chem 16:3966–3985. doi:10.2174/092986709789352277
Furmanowa M, Syklowska-Baranek K (2000) Hairy root cultures of Taxus x media var. Hicksii Rehd. as a new source of paclitaxel and 10-deacetylbaccatin III. Biotechnol Lett 22:683–686. doi:10.1007/s10529-005-0223-5
Huang YF, Lan WZ, Chen C, Yu LJ (2005) The role of lipoxygenase in elicitor-induced taxol production in Taxus chinensis cell cultures. Process Biochem 40:2793–2797. doi:10.1016/j.procbio.2004.12.018
Lamotte O, Courtois C, Barnavon L, Pugin A, Wendehenne D (2005) Nitric oxide in plants: the biosynthesis and cell signaling properties of a fascinating molecule. Planta 221(1):1–4. doi:10.1007/s00425-005-1494-8
Li G, Faibushevich A, Turunen BJ, Yoon SO, Georg G, Michaelis ML, Doborowsky RT (2003) Stabilization of the cyclin-dependent kinase 5 activator, p35, by paclitaxel decreases β-amyloidtoxicity in cortical neurons. J Neurochem 84:347–362. doi:10.1046/j.1471-4159.2003.01526.x
Li ST, Zhang M, Fu CI, Xie S, Zhang Y, Yu LY (2012) Molecular cloning and characterization of two 9-Lipoxygenase genes from Taxus chinensis. Plant Mol Biol Rep 30:1283–1290. doi:10.1007/s11105-012-0439-1
Misra AN, Misra M, Singh R (2010) Nitric oxide biochemistry, mode of action and signaling in plants. J Med Plants 4:2729–2739
Nims E, Dubois CP, Roberts SC, Walker EL (2006) Expression profiling of genes involved in paclitaxel biosynthesis for targeted metabolic engineering. Metab Eng 8:385–394. doi:10.1016/j.ymben.2006.04.001
Onrubia M, Moyano E, Bonfill M, Cusido RM, Goossens A, Palazon J (2012) Coronatine, a more powerful elicitor for inducing taxane biosynthesis in Taxus media cell cultures than methyl jasmonate. J Plant Physiol 170(2):211–219. doi:10.1016/j.jplph.2012.09.004
Oiao W, Li C, Fan LM (2014) Cross-talk between nitric oxide and hydrogen peroxide in plant responses to abiotic stresses. Environ Exp Bot 100:84–93. doi:10.1016/j.envexpbot.2013.12.014
Onrubia M, Cusido´ RM, Ramirez K, Hernandez-Vazquez L, Moyano E, Bonfill M, Palazon J (2013) Bioprocessing of plant in vitro systems for the mass production of pharmaceutically important metabolites: paclitaxel and its derivatives. Curr Med Chem 20:880–891. doi:10.2174/0929867311320070004
Palavan-Unsal N, Arisan D (2009) Nitric oxide signalling in plants. Bot Rev 75:203–229. doi:10.1007/s12229-009-9031-2
Sabater-Jara AB, Onrubia M, Moyano E, Bonfill M, Palazon J, Pedreño MA, Cusido RM (2014) Synergistic effect of cyclodextrins and methyl jasmonate on taxane production in Taxus x media cell cultures. Plant Biotechnol J 12(8):1075–1084. doi:10.1111/pbi.12214
Scheler C, Durner J, Astier J (2013) Nitric oxide and reactive oxygen species in plant biotic interactions. Curr Opin Plant Biol 16:534–539. doi:10.1016/j.pbi.2013.06.020
Sudha G, Ravishankar GA (2002) Involvement and interaction of various signaling compounds on the plant metabolic events during defense response, resistance to stress factors, formation of secondary metabolites and their molecular aspects. Plant Cell Tiss Organ Cult 71:181–212. doi:10.1023/A:1020379024347
Syklowska-Baranek K, Pietrosiuk A, Kokoszka A, Furmanowa M (2009) Enhancement of taxane production in hairy root culture of Taxus x media var. Hicksii. J Plant Physiol 166:1950–1954. doi:10.1016/j.jplph.2009.05.001
Sykłowska-Baranek K, Pietrosiuk A, Naliwajski MR, Kawiak A, Jeziorek M, Wyderska S, Łojkowska E, Chinou I (2012) Effect of l-phenylalanine on PAL activity and production of naphthoquinone pigments in suspension cultures of Arnebia euchroma (Royle) Johnst. In Vitro Cell Dev-Pl 48:555–564. doi:10.1007/s11627-012-9443-2
Sykłowska-Baranek K, Pilarek M, Bonfill M, Kafel K, Pietrosiuk A (2015) Perfluorodecalin-supported system enhances taxane production in hairy root cultures of Taxus x media var. Hicksii carrying a taxadiene synthase transgene. Plant Cell Tiss Organ Cult 120:1051–1059. doi:10.1007/s11240-014-0659-1
Tabata H (2004) Paclitaxel production by plant-cell-culture technology. Adv Biochem Engin Biotechnol 7:1–23. doi:10.1007/b13538
Tabata H (2006) Production of paclitaxel and the related taxanes by cell suspension cultures of Taxus species. Curr Drug Targets 7:453–461. doi:10.2174/138945006776359368
Theodoridis G, de Jong CF, Laskaris G, Verpoorte R (1998a) Application of SPE for the HPLC analysis of taxanes from Taxus cell cultures. Chromatographia 47:25–34. doi:10.1007/BF02466782
Theodoridis G, Laskaris G, de Jong CF, Hofte AJP, Verpoorte R (1998b) Determination of paclitaxel and related diterpenoids in plant extracts by high-performance liquid chromatography with UV detection in high-performance liquid chromatography-mass spectrometry. J Chromatogr A 802:297–305. doi:10.1016/S0021-9673(97)01174-6
Trapet P, Kulik A, Lamotte O, Jeandroz S, Bourque S, Nicolas-Francès V, Rosnoblet C, Besson-Bard A, Wendehenne D (2014) NO signaling in plant immunity: a tale of messengers. Phytochemistry 112:72–79. doi:10.1016/j.phytochem.2014.03.015
Wang JW, Wu JY (2004) Involvement of nitric oxide in elicitor-induced defense responses and secondary metabolism of Taxus chinensis cells. Nitric Oxide 11:298–306. doi:10.1016/j.niox.2004.10.003
Wang JW, Wu JY (2005) Nitric oxide is involved in methyl jasmonate-induced defense responses and secondary metabolism activities of Taxus cells. Plant Cell Physiol 46:923–930. doi:10.1093/pcp/pci098
Wang JW, Zheng LP, Wu JY, Tan RX (2006) Involvement of nitric oxide in oxidative burst, phenylalanine ammonia-lyase activation and Taxol production induced by low-energy ultrasound in Taxus yunnanensis cell suspension cultures. Nitric Oxide 15:351–358. doi:10.1016/j.niox.2006.04.261
Wang JW, Zheng LP, Tan RX (2007a) Involvement of nitric oxide in cerebroside-induced defense responses and taxol production in Taxus yunnanensis suspension cells. Appl Microbiol. doi:10.1007/s00253-007-0927-7
Wang YD, Wu JC, Yuan YJ (2007b) Salicylic acid-induced taxol production and isopentenyl pyrophosphate biosynthesis in suspension cultures of Taxus chinensis var. mairei. Cell Biol Int 31:1179–1183. doi:10.1016/j.cellbi.2007.03.038
Wasternack C (2015) Action of jasmonates in plant stress responses and development–applied aspects. Biotechnol Adv 32:31–39. doi:10.1016/j.biotechadv.2013.09.009
Wojtaszek P (2000) Nitric oxide in plants. To NO or not to NO. Phytochemistry 54:1–4. doi:10.1016/S0031-9422(00)00056-X
Wu J, Lin L (2003) Enhancement of taxol production and release in Taxus chinensis cell cultures by ultrasound, methyl jasmonate and in situ solvent extraction. Appl Microbiol Biotechnol 62:151–155. doi:10.1007/s00253-003-1275-x
Xiao WH, Cheng J, Yuan YJ (2009) Spatial-temporal distribution of nitric oxide involved in regulation of phenylalanine ammonialyase activation and Taxol production in immobilized Taxus cuspidata cells. J Biotechnol 139:222–228. doi:10.1016/j.jbiotec.2008.11.005
Xu M, Dong J, Zhu M (2004) Involvement of NO in fungal elicitor-induced activation of PAL and stimulation of taxol synthesis in Taxus chinenis suspension cells. Chin Sci Bull 49(10):1038–1043. doi:10.1360/04wc0067
Yu M, Lamattina L, Spoel SH, Loake GJ (2014) Nitric oxide function in plant biology: a redox cue in deconvolution. New Phytol 202(4):1142–1156. doi:10.1111/nph.12739
Zhang CH, Wu JY, He GY (2002) Effects of inoculum size and age on biomass growth and paclitaxel production of elicitor-treated Taxus yunnanensis cell cultures. Appl Microbiol Biotechnol 60:396–402. doi:10.1007/s00253-002-1130-5
Zhang C, Fevereiro P, He G, Chen Z (2007) Enhanced paclitaxel productivity and release capacity of Taxus chinensis cell suspension cultures adapted to chitosan. Plant Sci 172:158–163. doi:10.1016/j.plantsci.2006.08.002
Zhang B, Zheng LP, Wang JW (2012) Nitric oxide elicitation for secondary metabolite production in cultured plant cells. Appl Microbiol Biotechnol 93:455–466. doi:10.1007/s00253-011-3658-8
Zhong JJ (2002) Plant cell culture for production of paclitaxel and other taxanes. J Biosci Bioeng 94:591–599. doi:10.1016/S1389-1723(02)80200-6
Zucker M (1965) Induction of phenylalanine deaminase by light and its relation to chlorogenic acid synthesis in potato tuber tissue. Plant Physiol 40:779–784. doi:10.1104/pp.40.5.779
Acknowledgments
This study was supported by research grant No. N N405 362537 from the Polish Ministry of Science and Higher Education. Authors participated equally in the reported work and in preparation of this manuscript. Authors are very grateful for the help and technical assistance of Bożenna Sztyber and Alina Łukasiewicz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Communicated by A. Krolicka.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sykłowska-Baranek, K., Grech-Baran, M., Naliwajski, M.R. et al. Paclitaxel production and PAL activity in hairy root cultures of Taxus x media var. Hicksii carrying a taxadiene synthase transgene elicited with nitric oxide and methyl jasmonate. Acta Physiol Plant 37, 218 (2015). https://doi.org/10.1007/s11738-015-1949-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1949-x