Abstract
Enhanced taxane production was observed in a hybrid, two liquid phases containing, cultures of Taxus x media var. Hicksii hairy root carrying a taxadiene synthase transgene, supported with liquid perfluorodecalin (PFD) in degassed or aerated form. The hairy root cultures were elicited with methyl jasmonate (MJ, 100 μM) or coronatine (COR, 1 μM), and fed with sucrose and l-phenylalanine. The root growth was not stimulated by PFD addition, irrespective of the day of its application (day 0 and 14). However, in the cultures elicited with MJ and performed in the presence of PFD the final root biomass accumulation was higher than in cultures performed without PFD while the opposite effect was observed in cultures supplemented with COR. The highest paclitaxel content in root biomass was determined at the end of the cultures elicited with MJ and supplemented with PFD-degassed at day 0 or 14, 1,440.8 and 1,432.5 μg g−1 DW, respectively. The highest total (i.e. intracellular + extracellular: both in aqueous and PFD phases) paclitaxel yield in flasks (149.15 μg flask−1) was noted after the application of PFD-degassed at day 14. The other taxane detected was baccatin III, only in the root biomass, with the highest content (76.9 μg g−1 DW) observed under COR treatment. Although COR stimulated paclitaxel production with less efficiency than MJ, it resulted in higher paclitaxel excretion to the liquid phases of culture medium and PFD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taxanes are well known drug agents used in anti-cancer therapies against tumors of ovaries, breast, bladder and other human organs. Paclitaxel (PC), the first pharmaceutically applied taxane, can be extracted from the bark of yew trees but with very low efficiency. The utilization of PC and its semi-synthetic derivative docetaxel in anticancer therapy has led to the development of analogues, characterized by improved solubility and fewer side-effects (Expósito et al. 2009; Onrubia et al. 2013). The search for other alternative methods of biotechnological production of taxanes in plant cell, tissue and organ cultures is still fully economically justified and has been extensively discussed (Tabata 2004; Vongpaseuth and Roberts 2007; Frense 2007; Maheshwari et al. 2008; Expósito et al. 2009; Sabater-Jara et al. 2010; Malik et al. 2011; Onrubia et al. 2013).
To date, the most effective strategy for enhancing in vitro taxane production has been considered to be elicitation with methyl jasmonate (MJ), and immense effort has been invested in elucidating its molecular mechanism of action in yew cell suspension cultures, revealing the up-regulation of genes engaged in the taxane biosynthetic pathway (Nims et al. 2006; Onrubia et al. 2010, 2012; Lenka et al. 2012; Li et al. 2012; Sun et al. 2013).
Taxane production has also been substantially enhanced by metabolic engineering. The introduction of taxadiene synthase gene (txs transgene) encoding the enzyme taxadiene synthase, which performs the first committed cyclization step in the taxane biosynthetic pathway alone (Exposito et al. 2010), or together with the 10-deacetylbaccatin III-10β-O-acetyltransferase gene (dbat gene), encoding the enzyme which converts 10-deactylbaccatin III (10-DAB III) to baccatin III (Ho et al. 2005) have been reported. These metabolic engineering modifications resulted in significantly higher taxane accumulation in transgenic cell lines than in non-transgenic ones.
Our results from a previous study (Sykłowska-Baranek et al. 2014) clearly show that liquid perfluorochemical (synonym: perfluorocarbons; PFC) applied directly into the plant cell culture system constitutes a simple, flexible and efficient system of increasing secondary metabolite yield. To date, PFCs, that are fully synthetic, biochemically inert and immiscible with aqueous phase compounds, have been frequently applied in plant cell suspension cultures to obtain higher cell densities (Lowe et al. 2003; Davey et al. 2005; Pilarek and Szewczyk 2008) rather than to identify a positive influence on secondary metabolite excretion (Sykłowska-Baranek et al. 2014), similarly with hairy root cultures (Kanokwaree and Doran 1998). From the bioprocess engineering point of view, PFCs are recognized as the most flexible liquid carriers/scavengers of gaseous compounds (O2 and CO2, mainly) and were successfully applied in culture systems of various types of cells, i.e. microorganisms such as bacteria (Pilarek et al. 2011; 2013a), yeast (Pilarek et al. 2006), fungi (Elibol and Ozer 2000) and microalgae (Hillig et al. 2013, 2014), animal (Shiba et al. 1998; Rappaport 2003; Pilarek et al. 2013b, 2014; Douglas et al. 2014) as well as plant cells (Wardrop et al. 1996; Lowe et al. 2003; Davey et al. 2005; Pilarek and Szewczyk 2008; Sykłowska-Baranek et al. 2014).
The gas transfer rate into PFCs increases linearly with the partial pressure of a component in the gaseous phase (Castro and Briceno 2010; Sobieszuk and Pilarek 2012). Importantly, PFCs are immiscible with aqueous media and they create an auxiliary phase below the aqueous phase of the culture medium at the bottom of the culture flask/vessel. Due to these properties, in PFC-supported culture systems the auxiliary interfacial area (i.e. PFC/medium) for mass transfer appears independently of the typical medium/air-phase interface. Most recently, Perfluorodecalin (PFD) has been evaluated by Sykłowska-Baranek et al. (2014) as a suitable immiscible liquid for in situ extraction of naphthoquinones in cell suspension cultures of Arnebia euchroma, achieving a 50 % increase in alkannin/shikonin yield. To our knowledge, up to date, there is only one study on the PFC-supported culture system applied to hairy root cultures (Kanokwaree and Doran 1998). The authors used a polypropylene membrane tubing system and PFC-based emulsion to improve oxygen transfer in Atropa belladonna hairy roots bioreactor cultures, but the effect of PFC on atropine levels was not taken into consideration.
The aim of our current study was to demonstrate the effects of PFD, applied in the form of (1) PFD-degassed or (2) PFD-aerated, both on biomass growth and taxane production in hairy root cultures of Taxus x media var. Hicksii carrying the taxane synthase (txs) transgene of Taxus baccata. We found that elicitation, together with additional sucrose and precursor feeding in the PFD-supported hairy root culture systems enhanced taxane production without significant growth retardation. This is the first report on the use of liquid PFC as a taxane production-enhancing agent in yew cells or organ cultures in general, and specifically in Taxus spp. hairy roots carrying the txs transgene.
Materials and methods
Plant material
Seeds of Taxus x media var. Hicksii were collected from the Botanical Garden of the Polish Academy of Science in Powsin (Poland) and the in vitro plantlets were obtained from these seeds according to the method described by Zhiri et al. (1994) from isolated embryos. The plantlets subjected to the genetic transformation procedure were cultivated in vitro over 10 years with a transfer to fresh solid DCR-M medium every 8 weeks.
The hairy root cultures were obtained by direct inoculation of the Taxus x media var. Hicksii plantlets with the C58C1 strain of Agrobacterium tumefaciens carrying the plasmid RiA4 of A. rhizogenes and the binary plasmid pCAMBIA-TXS-His, harbouring the taxadiene synthase (txs) gene of T. baccata (GenBank accession: AY424738), under the control of the 35S CaMV promoter, and the hygromycin phosphotransferase gene (hptII) as a resistance marker. Putative transformed roots appeared in the wound sites 6–8 months after inoculation and when 2–3 cm long they were excised and cultured individually in liquid hormone-free modified DCR-M medium (Syklowska-Baranek et al. 2009) with 50 mg l−1 hygromicin and cefotaxime (Claforan) 500 mg l−1 for 2 weeks and subcultured twice at 4-week intervals. After removing the antibiotic, they were subcultured routinely every 4 weeks in 250 ml Erlenmeyer flasks with 35 ml hormone-free DCR-M medium. The cultures were maintained at 25 ± 1 °C in the dark on an INFORS AG TR 250 shaker (Switzerland) at 105 rpm.
The transformed nature of the root lines was confirmed by PCR analysis.
PCR analysis
The primer sequences corresponding to the txs gene were chosen using the BTI software Gene Tool Lite (version 1.0.0.1) and oligonucleotides for the genes were synthesized by TIB Molbiol (Berlin, Germany). The primers (Forward 5′-CCA CGG TTT CCT CAG GCC CTC AA-3′ and Reverse 5′-GCC GCC GAA TTT GTC CAG CAG AT-3′) were designed to amplify a band of 552 bp.
PCR was performed with a 2 μl gDNA template using puReTag Ready-To-Go PCR Beads (GE Healthcare, Buckinghamshire, UK) and was carried out in a programmable thermocycler (MiniCycler MJ Research, Watertown, MA). The gDNA was obtained using a DNeasyVR Plant Mini Kit (Qiagen, Hilden, Germany). The PCRs were performed under the following conditions: initial denaturation at 94 °C for 5 min, followed by 35 cycles at 94 °C for 30 s, 58 ºC for 30 s and 72 °C for 45 s and then a final extension at 72 °C for 5 min.
Perfluorodecalin
Perfluorodecalin (PFD, C10F18; 98 % equimolar mixture of cis-/trans- isomers; ABCR GmbH & Co. KG, Karlsruhe, Germany), the synthetic perfluorinated analog of decalin, was used in experiments. First, PFD was autoclaved at 121 °C for 20 min to ensure aseptic conditions and PFD-degassed. To obtain the PFD-aerated, it was aseptically saturated with atmospheric air for 15 min. according to the procedure described previously (Pilarek and Szewczyk 2008). Afterwards, 20 ml of PFD, degassed or aerated, was added to 35 ml of sterile culture medium. PFD was applied to the flasks on the day of inoculation (day 0) or at day 14 of culture. After addition of PFD to the medium, all flasks were closed with tightly squeezed aluminum caps.
Elicitor and precursor feeding
We studied the effect of the precursors l-phenylalanine (PHE; 100 μM, Sigma-Aldrich) and sucrose (S; 30 g l−1, POCH, Poland) and the elicitors methyl jasmonate (MJ; 100 μM, Sigma-Aldrich) and coronatine (COR; 1 μM, Sigma-Aldrich) added at day 28 of culture of the hairy roots growing in Erlenmeyer flasks containing 35 ml DCR-M, with or without a PFD phase in degassed or aerated form. The two precursors were added together in all the experiments (with exception of the control culture), and the elicitors were assayed separately.
PHE and sucrose were autoclaved prior to use and added to the media in the laminar flow cabinet. MJ and COR were dissolved in EtOH (POCH, Poland) aseptically and applied to the cultures after autoclaving.
-
Each flask was inoculated with 0.5 ± 0.05 g fresh weight (FW) of roots
-
The following culture systems were performed
(1) Control culture—without any supplementation; (2) with MJ; (3) with COR; (4) with PFD-degassed added at day 0 without any elicitor; (5) with PFD-degassed added on day 0 plus MJ; (6) with PFD-degassed added at day 0 plus COR; (7) with PFD-aerated added at day 0 without any elicitor; (8) with PFD-aerated added at day 0 plus MJ; (9) with PFD-aerated added at day 0 plus COR; (10) with PFD-degassed added at day 14 without any elicitor; (11) with PFD-degassed added at day 14 plus MJ; (12) with PFD-degassed added at day 14 plus COR; (13) with PFD-aerated added at day 14 without any elicitor; (14) with PFD-aerated added at day 14 plus MJ; (15) with PFD-aerated added at 14 day plus COR.
Samples from all considered culture systems (1–15) were harvested on days 28 (the same day of precursor and/or elicitor feeding), 35 and 42 of culture (representing 7 and 14 days after elicitation).
The fresh biomass increase was expressed as a ratio of final weight to initial weight. Roots and liquids from harvested samples were separated using a Büchner funnel. Roots were then gently pressed on filter paper to remove excess medium and their FW was recorded as well as dry weight (DW) after lyophilization.
Chemical analysis
The content of six taxanes: PC, taxol C, 10-deacetyltaxol, 7-epi-10-deacetyltaxol, baccatin III and 10-deacetylbaccatin III was determined in powdered dry tissue of transgenic roots, as well as in medium and PFD samples. Prior to extraction, phases of medium and PFD were placed into the separatory funnel and carefully separated. The PFD phase was directly washed twice with methanol. Methanolic extracts were combined and evaporated to dryness under reduced pressure. Next the PFD phase treated with 100 % MeOH (methanol) was regenerated by twice washing with distilled water and re-used in subsequent experiments.
The cleaning of samples taken from root tissue and the aqueous phase of the medium was performed using the method described by Theodoridis et al. (1998a). The residues arising from SPE (Solid Phase Extraction) extraction (root tissue and aqueous phase of medium) and the PFD phases were re-dissolved in 100 % MeOH (400 μl) and 20 μl underwent High Performance Liquid Chromatography with Diode Array Detection (HPLC–DAD) analysis using the DIONEX (USA) system with a UVD 340S diode array detector and an automated sample injector (ASI-100). For compound separation, a Kinetex (Phenomenex, USA) 100 × 4.60 mm column was eluted employing the gradient program as described by Theodoridis et al. (1998b). The DAD spectrophotometer was set at a wavelength range of 215 to 275 nm. The taxanes were identified and quantified at 227 nm. The peaks were assigned by spiking the samples with the standards and comparing the retention times and UV spectra.
The standard compounds were produced by CHOMADEX (USA) and purchased from LCG Standards (Poland). All chemicals were of HPLC-grade and purchased from Sigma-Aldrich.
All experiments were performed in triplicate. The statistical significance between means was assessed using analysis of variance (ANOVA) and Tukey’s multiple range test. A probability of p = 0.05 was considered significant.
Results and discussion
Hairy root growth
The txs transgene of T. baccata was successfully inserted into T. x media var. Hicksii tissue and two transgenic root lines carrying the txs gene were established, they were described as: ATMA and ATM (Fig. 1). Line ATMA demonstrated good and stable growth in hormone-free liquid DCR-M medium and was chosen for further investigation.
In two-step hairy root cultures, firstly the influence on hairy root biomass accumulation of PFD-degassed or PFD-aerated applied to the media on the day of inoculation (day 0) or at day 14 of culture was evaluated by comparison with the control (without any additives). In the next step, the cultures with PFD phases added to the medium at day 0 or 14 were supplemented with the elicitors MJ or COR at day 28 and cultivated a further 14 days, harvesting samples at days 35 and 42. All types of elicited cultures were supplemented with additional sucrose and PHE at day 28 of culture.
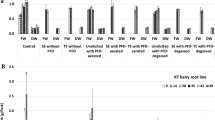
The hairy root growth increase (expressed as a ratio of final FW/initial FW) obtained under these conditions was compared with the root biomass increase in the additive-free control culture and with results from cultures supplemented with elicitors, additional sucrose and PHE but without any PFD phases (Fig. 2).
The highest final fresh biomass accumulation (1.9-fold ±0.21) was observed in the control after 6 weeks of culture. In PFD-supported cultures, higher root growth was observed with PFD-degassed, and it was significantly (p = 0.05) higher when applied at day 14 of culture than day 0 (Fig. 2). In fact, the root biomass increase in presence of PFD-degassed added at day 14 was not significantly (p = 0.05) lower than in the control (1.85-fold ±0.55). The effect of PFD-aerated on root growth was detrimental, irrespective of the day of its application, but root biomass accumulation was significantly (p = 0.05) lower at the end of culture when it was applied at day 0 (1.1-fold ±0.28) than at day 14 (1.4-fold ±0.24).
Similarly, Kanokwaree and Doran (1998) did not note any growth improvement in A. belladonna hairy root cultures maintained in a bioreactor and supported with PFC-based emulsion. The ineffectiveness of PFC-mediated gas transfer into root clumps was attributed to PFC properties that allowed the enhancement of gas–liquid oxygen transfer but not liquid–solid.
When elicitors were added to the medium, at the end of second week of elicitation the hairy root growth was inhibited in comparison to the control culture to 1.3-fold ±0.02 and 1.6-fold ±0.42 in presence of MJ and COR, respectively. In the cultures supplemented with PFD-degassed or PFD-aerated at day 0, a significantly (p = 0.05) higher final root biomass was achieved under COR than MJ treatment. In contrast, when PFD phases were applied at day 14, final root growth was reduced to a greater extent after addition of MJ than COR, and this difference was more pronounced with PFD-aerated and elicitors.
The reversal of the detrimental effect of elicitation on biomass growth could be attributed to the lack of direct contact between root tissue and elicitors as well as the metabolites produced by the roots, including PC, due to compound transfer into the perfluorinated phase and the consequent reduction in toxicity. This assumption is supported by the observation that not only gasses but also solid compounds can dissolve in PFCs, as indicated by the results of the current study and our previous report (Sykłowska-Baranek et al. 2014), and also described for pulmonary drug delivery systems by Lehmler (2007). Moreover, the toxic effect of PC on cell viability in plant cell in vitro cultures has also been reported by Kim et al. (2005), Expósito et al. (2009).
In suspension cultures of Taxus x media (Exposito et al. 2010), when comparing the fresh biomass accumulation of non-transformed (control) with two transformed cell lines, one carrying an additional txs gene (TXS line) apart from rol genes (RolC line), only a slight growth suppression was noted after MJ elicitation in the transformed cultures. However, the TXS line was more sensitive to the MJ supplementation than RolC and showed a weaker growth.
Onrubia et al. (2012) investigated for the first time the potential of COR versus MJ to enhance taxane production, also in suspension cultures of Taxus x media carrying the additional txs gene. The fresh biomass formation was not considerably diminished by COR-treatment (3.5-fold) compared to the control (threefold). The cell growth obtained in MJ-treated cultures was significantly lower, amounting to twofold at the end of the experiment (day 28) (Onrubia et al. 2012).
In our previous reports on Taxus x media var. Hicksii hairy root cultures obtained by transformation with the wild strain of A. rhizogenes LBA 9402 (line KT), twofold biomass reduction was observed under MJ elicitation (Furmanowa and Syklowska-Baranek 2000; Syklowska-Baranek et al. 2009).
In transgenic suspension cultures of T. mairei carrying txs and dbat inserts a 20 % decrease in cell growth rate was caused by MJ elicitation (Ho et al. 2005). The considerable differences in elicitor effects on Taxus spp. tissue growth that have been reported and reviewed (Tabata 2004; Vongpaseuth and Roberts 2007; Onrubia et al. 2010) indicate that the responses depend on elicitor type, dose and duration of elicitation, cell line and its state of development.
As the best root growth was observed in control cultures, it could be inferred that medium supplementation with extra sucrose and PHE at day 28 did not negatively affect biomass accumulation. Additional carbohydrates applied to Taxus spp. suspension cultures have had beneficial effects on both biomass accumulation and taxane content (Srinivasan et al. 1995; Ketchum and Gibson 1996; Luo and He 2004).
Taxane accumulation
Among the seven taxanes searched for in the txs transgenic root cultures of T. x media var. Hicksii, only PC and baccatin III were found, and the latter only in root biomass. No taxanes were determined in the control or unelicited cultures supplemented with a PFD-phase, neither in the root biomass, nor the medium or PFD-phase, irrespective of when the phase was applied. The addition of extra S and PHE without elicitors had no effect on taxane accumulation. Very low levels of taxane biosynthetic genes have been observed in unelicited suspension cultures of Taxus spp, where only 10-DAB III was detected (Nims et al. 2006). In a previous study with Taxus x media var. Hicksii, in a control culture of the hairy root line KT, of the three target taxanes, 10-DAB III, baccatin III and PC, we only detected the latter at a concentration of 11.6 μg g−1 DW (Syklowska-Baranek et al. 2009). When 100 μM PHE was added to the medium without MJ, there was no taxane accumulation or excretion throughout the culture period (Syklowska-Baranek et al. 2009). In transgenic suspension cultures of T. x media cells carrying the txs transgene maintained in a two-stage culture, taxane content in growth-promoting medium was very low, irrespective of the cell line (untransformed, RolC and TXS), and increased significantly after cell transfer to the production-promoting medium, with a taxane yield twofold higher in untransformed cells (2.2 mg l−1) than in transgenic lines (Expósito et al. 2009). In a subsequent study, a sixfold rise in taxane content was noted (up to 8.8 mg l−1) after txs transgene-carrying cells were transferred to the production medium (Onrubia et al. 2012).
Medium supplementation with MJ or COR in our experiments induced taxane production. Elicitors were added at day 28 of root growth, alone or with the degassed or aerated PFD-phases applied on the day of inoculation (day 0) or day 14 of culture (day 14). In all types of culture, the results of elicitation were recorded at days 35 (7 days after elicitor addition) and 42 (14 days after elicitor addition), as maximal taxane accumulation can last for 2–15 days (Nims et al. 2006).
MJ turned out to be more efficient than COR for PC production, while COR was a better inducer of baccatin III (Fig. 3). Baccatin III was determined only intracellularly. PC total content (intracellular + medium phase) was almost threefold higher in the presence of MJ than COR after 7 days of elicitation: 43.82 ± 5.48 compared to 15.2 ± 4.89 μg flask−1, respectively. After 14 days, this disproportion had diminished and the PC amount was only 1.2-fold higher in MJ- than COR-treated roots: 56.67 ± 6.67 μg flask−1 versus 44.0 ± 9.84 μg flask−1, respectively (Fig. 3a, b). In presence of MJ, PC was also detected in the medium at similar levels (not significantly different at p = 0.05) after 7 and 14 days of elicitation: 9.99 ± 0.012 and 11.2 ± 0.32 μg flask−1, respectively. Under COR, PC excretion was increased over twofold, from 9.3 ± 0.65 to 17.8 ± 0.37 μg flask−1.
PC content (μg flask−1) in Taxus x media var. Hicksii txs transgene-carrying hairy root cultures maintained in culture systems supported with PFD-degassed or PFD-aerated and elicited with MJ or COR: a after 1 week of elicitation; b after 2 weeks of elicitation. Data are the mean of three independent replicates ±SE
In all cultures supported with PFD-phases, COR addition entailed a significantly lower accumulation of PC in root biomass than MJ (Table 1; Fig. 3a, b) at the end of the culture period. The highest PC yield, but not significant different at p = 0.05, was determined in cultures supplemented with MJ and PFD-degassed on the day of inoculation (day 0) or at day 14 of culture: 1,440.8 ± 92.21 and 1,432.5 ± 108.69 μg g−1 DW, respectively. In MJ-elicited cultures, the PC content was significantly lower with PFD-aerated than PFD-degassed, regardless of the time of addition. In COR-elicited cultures the PC content was more influenced by the time of PFD-phase addition than the level of air-saturation. When PFD-degassed was employed at day 0, the PC accumulation was twofold less than when added at day 14, while PFD-aerated resulted in a 1.7-fold reduction at the same conditions.
Regarding extracellular PC, COR stimulated its excretion into both medium and PFD phases to a greater extent than MJ (Fig. 3a, b). Under COR, the highest extracellular PC content in the medium (16.6 ± 0.65 μg flask−1) and PFD-phase (20.1 ± 0.49 μg flask−1) was noted at the end of the culture (2 weeks after elicitation) in cultures supported with PFD-degassed at day 0 (Fig. 3b). The rate of taxane excretion, calculated as total extracellular taxane content divided by total intra- and extracellular taxane content, was significantly higher in presence of COR at the end of the culture (day 42). Additionally, when PC was detected in both the medium and PFD phases, its concentration was higher in the latter (Fig. 3b).
The second determined taxane was baccatin III, which was detected only in dry biomass of hairy roots and in almost all cases at the end of the culture (Table 1). COR was more efficient in stimulating its production than MJ, causing a 64-fold higher baccatin III accumulation than MJ. Medium supplemented with PFD-phases resulted in similar (not significantly different at p = 0.05) levels of baccatin III when this compound was detected. Among all tested culture system modifications with PFD, significantly higher amounts of baccatin III were noted in those with PFD-degassed applied at day 14 (Table 1).
MJ proved to be more efficient in inducing taxane production than COR. Onrubia et al. (2012) reported differences in the time course of taxane formation in MJ and COR-treated suspension cultures of Taxus media cells carrying the txs transgene. The total taxane content peaked at day 12 and was significantly lower than in the presence of COR. The main taxane was baccatin III. This pattern was not observed in the current work. The highest taxane yield was noted after 14 days of elicitation and baccatin III was only detected in the root biomass and at lower levels than PC. However, the baccatin III yield was significantly higher under treatment with COR than MJ in cultures without PFD-phases. The differences in response to the elicitor treatment between cell suspension and hairy root cultures, both carrying the txs transgene, could be because unorganized cells and cell formation in specialized tissues are governed by different factors.
Previous investigations on Taxus x media var. Hicksii hairy roots achieved by transformation with A. rhizogenes LBA 9402 and cultivated in medium supplemented only with 100 μM PHE revealed no taxanes in the root biomass until this precursor was simultaneously applied with MJ. The highest PC content (319.7 μg g−1 DW) was determined after 2 weeks of elicitation (Syklowska-Baranek et al. 2009). Under the conditions of the present experiments, in hairy roots carrying the txs transgene, 2 weeks of elicitation with MJ combined with extra S and PHE addition led to a PC level of 408.9 μg g−1 DW. The application of PFD-degassed at days 0 or 14 of culture and extra sucrose and PHE at day 28 caused a 3.5-fold rise in PC content, up to 1,440.8 and 1,432.5 μg g−1 DW, respectively, compared to the control.
Exposito et al. (2010) demonstrated that txs transgene expression and taxane accumulation in all their untransformed cell lines, either carrying only rol genes (Rol C line) and another carrying rol genes together with the txs transgene (TXS line) was clearly dependent on MJ action. Moreover, the TXS line clearly exceeded the Rol C line in capacity for taxane production.
Our results also seem to be supported on a molecular level by earlier findings by Onrubia et al. (2010), who reported a transient increase in the expression of txs and bapt genes in the presence of MJ, followed by a significantly enhanced production of PC and baccatin III.
Conclusions
This is the first report on the use of liquid PFC as an agent to enhance PC production in Taxus in vitro cultures, specifically in cultures of Taxus hairy roots carrying a txs transgene under the control of the 35S CaMV promoter. Our PFD-supported culture system elicited with MJ promoted PC production without any significant elicitor-associated growth reduction. In some experimental conditions, PC was determined both in the aqueous phase of the culture medium and phase of PFD, with higher levels dissolved in the latter. COR stimulated PC production with lower efficiency than MJ but caused greater excretion of this compound.
The results of our investigation suggest that a system harnessing liquid PFCs has potential for the enhancement of PC and other taxane productivity via Taxus spp. in vitro cultures.
References
Castro CI, Briceno JC (2010) Perfluorocarbon-based oxygen carriers: review of products and trials. Artif Organs 34:622–634. doi:10.1111/j.1525-1594.2009.00944.x
Davey MR, Anthony P, Power JB, Lowe KC (2005) Plant protoplast technology: current status. Acta Physiol Plant 27:117–129. doi:10.1007/s11738-005-0044-0
Douglas TEL, Pilarek M, Kalaszczyńska I, Senderek I, Skwarczyńska A, Cuijpers VMJI, Modrzejewska Z, Lewandowska-Szumieł M, Dubruel P (2014) Enrichment of chitosan hydrogels with perfluorodecalin promotes gelation and stem cell vitality. Mat Lett 128(2014):79–84. doi:10.1016/j.matlet.2014.03.173
Elibol M, Ozer D (2000) Influence of oxygen transfer on lipase production by Rhizopus arrhizus. Process Biochem 36:325–329. doi:10.1016/S0032-9592(00)00226-0
Exposito O, Syklowska-Baranek K, Moyano E, Onrubia M, Bonfill M, Palazon J, Cusido R (2010) Metabolic responses of Taxus media transformed cell cultures to the addition of methyl jasmonate. Biotechnol Prog 26:1145–1153. doi:10.1002/btpr.424
Expósito O, Bonfill M, Onrubia M, Jane A, Moyano E, Cusido RM, Palazon J, Pinol T (2009) Effect of taxol feeding on taxol and related taxane production in Taxus baccata suspension cultures. N Biotechnol 25:252–259. doi:10.1016/j.nbt.2008.11.001
Frense D (2007) Taxanes: perspectives for biotechnological production. Appl Microbiol Biotech 73:1233–1240. doi:10.1007/s00253-006-0711-0
Furmanowa M, Sykłowska-Baranek K (2000) Hairy root cultures of Taxus × media var. Hicksii Rehd. as a new source of paclitaxel and 10-deacetylbaccatin III. Biotechnol Lett 22:683–686. doi:10.1007/s10529-005-0223-5
Hillig F, Annemüller S, Chmielewska M, Pilarek M, Junne S, Neubauer P (2013) Bioprocess development in single-use systems for heterotrophic marine microalgae. Chem Ing Technik 85:153–161. doi:10.1002/cite.201200143
Hillig F, Pilarek M, Junne S, Neubauer P (2014) Cultivation of marine microorganisms in single-use systems. Adv Biochem Eng/Biotechnol 138:179–206. doi:10.1007/10_2013_219
Ho C, Chang S, Lung J (2005) The strategies to increase taxol production by using Taxus mairei cells transformed with TS and DBAT genes. Int J 3(3):179–185
Kanokwaree K, Doran P (1998) Application of membrane tubing aeration and perfluorocarbon to improve oxygen delivery to hairy root cultures. Biotechnol Prog 14:479–486. doi:10.1021/bp9800283
Ketchum REB, Gibson DM (1996) Paclitaxel production in suspension cell cultures of Taxus. Plant Cell Tiss Organ Cult 44:9–16. doi:10.1007/BF00039691
Kim BJ, Gibson DM, Shuler ML (2005) Relationship of viability and apoptosis to taxol production in Taxus sp. suspension cultures elicited with methyl jasmonate. Biotechnol Prog 21:700–707. doi:10.1021/bp050016z
Lehmler H-J (2007) Perfluorocarbon compounds as vehicles for pulmonary drug delivery. Expert Opin Drug Deliv 4:247–262. doi:10.1517/17425247.4.3.247
Lenka SK, Boutaoui N, Paulose B, Vongpaseuth K, Normalny J, Roberts SC, Walker EL (2012) Identification and expression analysis of methyl jasmonate responsive ESTs in paclitaxel producing Taxus cuspidata suspension culture cells. BMC Genom 13:148. doi:10.1186/1471-2164-13-148
Li S, Zhang P, Zhang M, Fu C, Zhao C, Dong Y, Guo A, Yu L (2012) Transcriptional profile of Taxus chinensis cells in response to methyl jasmonate. BMC Genom 13:295. doi:10.1186/1471-2164-13-295
Lowe KC, Anthony P, Power JB, Davey MR (2003) Novel approaches for regulating gas supply to plant systems in vitro: application and benefits of artificial gas carriers. In Vitro Cell Dev Biol-Plant 39:57–566. doi:10.1079/IVP2003469
Luo J, He G-Y (2004) Optimization of elicitors and precursors for paclitaxel production in cell suspension culture of Taxus chinensis in the presence of nutrient feeding. Process Biochem 39:1073–1079. doi:10.1016/S0032-9592(03)00219-X
Maheshwari P, Garg S, Kumar A (2008) Taxoids: biosynthesis and in vitro production. Biotechnol Mol Biol Rev 3:71–87. Article number-3ECD36340227
Malik S, Cusido RM, Mirjalili MH, Moyano E, Palazon J, Bonfill M (2011) Production of the anticancer drug taxol in Taxus baccata suspension cultures: a review. Process Biochem 46:23–34. doi:10.1016/j.procbio.2010.09.004
Nims E, Dubois CP, Roberts SC, Walker EL (2006) Expression profiling of genes involved in paclitaxel biosynthesis for targeted metabolic engineering. Metab Eng 8:385–394. doi:10.1016/j.ymben.2006.04.001
Onrubia M, Moyano E, Bonfill M, Exposito O, Palazon J, Cusido RM (2010) An approach to the molecular mechanism of methyl jasmonate and vanadyl sulphate elicitation in Taxus baccata cell cultures: the role of txs and bapt gene expression. Biochem Eng J 53:104–111. doi:10.1016/j.bej.2010.10.001
Onrubia M, Moyano E, Bonfill M, Cusido RM, Goossens A, Palazon J (2012) Coronatine, a more powerful elicitor for inducing taxane biosynthesis in Taxus media cell cultures than methyl jasmonate. J Plant Physiol 170(2):211–219. doi:10.1016/j.jplph.2012.09.004
Onrubia M, Cusidó RM, Ramirez K, Hernandez-Vazquez L, Moyano E, Bonfill M, Palazon J (2013) Bioprocessing of plant in vitro systems for the mass production of pharmaceutically important metabolites: paclitaxel and its derivatives. Curr Med Chem 20:880–891. doi:10.2174/0929867311320070004
Pilarek M, Szewczyk KW (2008) Effects of perfluorinated oxygen carrier application in yeast, fungi and plant cell suspension cultures. Biochem Eng J 41:38–42. doi:10.1016/j.bej.2008.03.004
Pilarek M, Szewczyk KW, Stępniewski J, Anderszewska A (2006) The use of a perfluorinated oxygen vector in cultures of microorganisms. Przemysł Chemiczny 85:1131–1133
Pilarek M, Glazyrina J, Neubauer P (2011) Enhanced growth and recombinant protein production of Escherichia coli by a perfluorinated oxygen carrier in miniaturized fed-batch cultures. Microb Cell Fact 10:50. doi:10.1186/1475-2859-10-50
Pilarek M, Brand E, Hillig F, Krause M, Neubauer P (2013a) Enhanced plasmid production in miniaturized high-cell-density cultures of Escherichia coli supported with perfluorinated oxygen carrier. Bioprocess Biosyst Eng 36:1079–1086. doi:10.1007/s00449-012-0861-7
Pilarek M, Grabowska I, Ciemerych MA, Dąbkowska K, Szewczyk KW (2013b) Morphology and growth of mammalian cells in a liquid/liquid culture system supported with oxygenated perfluorodecalin. Biotechnol Lett 35:387–1394. doi:10.1007/s10529-013-1218-2
Pilarek M, Grabowska I, Senderek I, Wojasinski M, Janicka J, Janczyk-Ilach K, Ciach T (2014) Liquid perfluorochemical-supported hybrid cell culture system for proliferation of chondrocytes on fibrous polylactide scaffolds. Bioprocess Biosyst Eng 37:1707–1715. doi:10.1007/s00449-014-1143-3
Rappaport C (2003) Progress in concept and practice of growing anchorage-dependent mammalian cells in three dimension. In Vitro Cell Dev Biol-Animal 39:187–192. doi:10.1290/1543-706X(2003)039<0187:RICAPO>2.0.CO;2
Sabater-Jara AB, Tudela LR, López-Pérez AJ (2010) In vitro culture of Taxus sp.: strategies to increase cell growth and taxoid production. Phytochem Rev 9:343–356. doi:10.1007/s11101-010-9167-z
Shiba Y, Ohshima T, Sato M (1998) Growth and morphology of anchorage-dependent animal cells in a liquid/liquid interface system. Biotechnol Bioeng 57:583–589. doi:10.1002/(SICI)1097-0290(19980305)57:5<583:AID-BIT10>3.0.CO;2-D
Sobieszuk P, Pilarek M (2012) Absorption of CO2 into perfluorinated gas carrier in the Taylor gas—liquid flow in a microchannel system. Chem Process Eng 33:595–602. doi:10.2478/v10176-012-0049-3
Srinivasan V, Pestchanker L, Moser S, Hirasuna TJ, Taticek RA, Shuler ML (1995) Taxol production in bioreactors: kinetics of biomass accumulation, nutrient uptake, and taxol production by cell suspension of Taxus baccata. Biotechnol Bioeng 47:666–676. doi:10.1002/bit.260470607
Sun G, Yang Y, Xie F, Wen J-F, Wu J, Wilson IW, Tang Q, Liu H, Qiu D (2013) Deep sequencing reveals transcriptome re-programming of Taxus × media cells to the elicitation with methyl jasmonate. PLoS One 8:e62865. doi:10.1371/journal.pone.0062865
Syklowska-Baranek K, Pietrosiuk A, Kokoszka A, Furmanowa M (2009) Enhancement of taxane production in hairy root culture of Taxus x media var Hicksii. J Plant Physiol 166:1950–1954. doi:10.1016/j.jplph.2009.05.001
Sykłowska-Baranek K, Pilarek M, Cichosz M, Pietrosiuk A (2014) Liquid perfluorodecalin application for in situ extraction and enhanced naphthoquinones production in Arnebia euchroma cell suspension cultures. Appl Biochem Biotechnol 172:2618–2627. doi:10.1007/s12010-013-0701-5
Tabata H (2004) Paclitaxel production by plant-cell-culture technology. Adv Biochem Engin/Biotechnol 7:1–23. doi:10.1007/b13538
Theodoridis G, de Jong CF, Laskaris G, Verpoorte R (1998a) Application of SPE for the HPLC analysis of taxanes from Taxus cell cultures. Chromatographia 47:25–34. doi:10.1007/BF02466782
Theodoridis G, Laskaris G, de Jong CF, Hofte AJP, Verpoorte R (1998b) Determination of paclitaxel and related diterpenoids in plant extracts by high-performance liquid chromatography with UV detection in high-performance liquid chromatography-mass spectrometry. J Chromatogr A 802:297–305. doi:10.1016/S0021-9673(97)01174-6
Vongpaseuth K, Roberts SC (2007) Advancements in the understanding of paclitaxel metabolism in tissue culture. Curr Pharm Biotechnol 8:219–236. doi:10.2174/138920107781387393
Wardrop J, Lowe KC, Power JB, Davey MR (1996) Perfluorochemicals and plant biotechnology: an improved protocol for protoplast culture and plant regeneration in rice (Oryza sativa L.). J Biotechnol 50:47–54. doi:10.1016/0168-1656(96)01548-9
Zhiri A, Jaziri M, Homès J, Vanhaelen M, Shimomura K (1994) Factors effecting the in vitro rapid germination of Taxus embryos and the evaluation of taxol content in the plantlets. Plant Cell, Tiss Org 39:261–263. doi:10.1007/BF00035979
Acknowledgments
This study, except the participation of Dr. Maciej Pilarek, was supported by research Grant No. N N405 362537 from the Polish Ministry of Science and Higher Education. Work in the Plant Physiology Laboratory (University of Barcelona) was financially supported by the Spanish MEC (BIO2011-29856-C02-1).
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is dedicated to the memory of Professor Dr. Dr. h.c. Mirosława Furmanowa.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Sykłowska-Baranek, K., Pilarek, M., Bonfill, M. et al. Perfluorodecalin-supported system enhances taxane production in hairy root cultures of Taxus x media var. Hicksii carrying a taxadiene synthase transgene. Plant Cell Tiss Organ Cult 120, 1051–1059 (2015). https://doi.org/10.1007/s11240-014-0659-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0659-1