Abstract
The soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) play essential roles in intracellular trafficking. However, few experimental data have clarified their roles in the stress responses and the early secretary pathway in Arabidopsis. The AtSec20 gene encodes a protein that is homologous to yeast Sec20p and mammalian BNIP1, which are involved in the Golgi-to-ER retrograde trafficking in yeast and mammalian cells. In this study, AtSec20 is found to be required for the responses to salt stress, osmotic stress and gibberellin (GA) during seed germination and early seedling establishment. Mutation of AtSec20 unaffects the morphology of intracellular organelles, such as endoplasmic reticulum (ER), trans-Golgi network, and peroxisome, and vacuolar protein trafficking is normal in sec20 mutants. Collectively, these results imply that the AtSec20 is involved in abiotic stress tolerance, potentially via roles in retrograde vesicle fusion process in Arabidopsis.




Similar content being viewed by others
References
Andag U, Schmitt HD (2003) Dsl1p, an essential component of the Golgi–endoplasmic reticulum retrieval system in yeast, uses the same sequence motif to interact with different subunits of the COPI vesicle coat. J Biol Chem 278:51722–51734
Andag U, Neumann T, Schmitt HD (2001) The coatomer-interacting protein Dsl1p is required for Golgi-to-endoplasmic reticulum retrieval in yeast. J Biol Chem 276:39150–39160
Aoki T, Kojima M, Tani K, Tagaya M (2008) Sec22b-dependent assembly of endoplasmic reticulum Q-SNARE proteins. Biochem J 410:93–100
Aoki T, Ichimura S, Itoh A, Kuramoto M, Shinkawa T, Isobe T, Tagaya M (2009) Identification of the neuroblastoma-amplified gene (NAG) product as a component of the syntaxin 18 complex implicated in Golgi-to-endoplasmic reticulum retrograde transport. Mol Biol Cell 20:2639–2649
Arasaki K, Taniguchi M, Tani K, Tagaya M (2006) RINT-1 regulates the localization and entry of ZW10 to the syntaxin 18 complex. Mol Biol Cell 17:2780–2788
Bassham DC, Blatt MR (2008) SNAREs: cogs and coordinators in signaling and development. Plant Physiol 147:1504–1515
Bassham DC, Brandizzi F, Otegui M, Sanderfoot AA (2008) The secretory system of Arabidopsis. In: Somerville CR, Meyerowitz EM (eds) the Arabidopsis Book. vol 6. American Society of Plant Biologists, Rockville, MD, pp e0116
Bassham DC, Sanderfoot AA, Kovaleva V, Zheng H, Raikhel NV (2000) AtVPS45 complex formation at the trans-Golgi network. Mol Biol Cell 11:2251–2265
Batoko H, Zheng HQ, Hawes C, Moore I (2000) A Rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 12:2201–2217
Bubeck J, Scheuring D, Hummel E, Langhans M, Viotti C, Foresti O, Denecke J, Banfield DK, Robinson DG (2008) The syntaxins SYP31 and SYP81 control ER–Golgi trafficking in the plant secretory pathway. Traffic 9:1629–1652
Cai H, Reinisch K, Ferro-Novick S (2007) Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell 12:671–682
Dilcher M, Veith B, Chidambaram S, Hartmann E, Schmitt HD, Fischer von Mollard G (2003) Use1p is a yeast SNARE protein required for retrograde traffic to the ER. EMBO J 22:3664–3674
Ebine K, Okatani Y, Uemura T, Goh T, Shoda K, Niihama M, Morita MT, Spitzer C, Otegui MS, Nakano A, Ueda T (2008) A SNARE complex unique to seed plants is required for protein storage vacuole biogenesis and seed development of Arabidopsis thaliana. Plant Cell 20:3006–3021
Hamaji K, Nagira M, Yoshida K, Ohnishi M, Oda Y, Uemura T, Goh T, Sato MH, Morita MT, Tasaka M, Hasezawa S, Nakano A, Hara-Nishimura I, Maeshima M, Fukaki H, Mimura T (2009) Dynamic aspects of ion accumulation by vesicle traffic under salt stress in Arabidopsis. Plant Cell Physiol 50:2023–2033
Hwang I (2008) Sorting and anterograde trafficking at the Golgi apparatus. Plant Physiol 148:673–683
Iinuma T, Aoki T, Arasaki K, Hirose H, Yamamoto A, Samata R, Hauri HP, Arimitsu N, Tagaya M, Tani K (2009) Role of syntaxin 18 in the organization of endoplasmic reticulum subdomains. J Cell Sci 122(Pt 10):1680–1690
Jahn RC, Scheller RH (2006) SNAREs-engines for membrane fusion. Nat Rev Mol Cell Biol 7:631–643
Kim SJ, Bassham DC (2011) TNO1 is involved in salt tolerance and vacuolar trafficking in Arabidopsis. Plant Physiol 156:514–526
Kraynack BA, Chan A, Rosenthal E, Essid M, Umansky B, Waters MG, Schmitt HD (2005) Dsl1p, Tip20p, and the novel Dsl3(Sec39) protein are required for the stability of the Q/t–SNARE complex at the endoplasmic reticulum in yeast. Mol Biol Cell 16:3963–3677
Lee CF, Pu HY, Wang LC, Sayler RJ, Yeh CH, Wu SJ (2006) Mutation in a homolog of yeast Vps53p accounts for the heat and osmotic hypersensitive phenotypes in Arabidopsis hit1-1 mutant. Planta 224:330–338
Leshem Y, Melamed-Book N, Cagnac O, Ronen G, Nishri Y, Solomon M, Cohen G, Levine A (2006) Suppression of Arabidopsis vesicle-SNARE expression inhibited fusion of H2O2-containing vesicles with tonoplast and increased salt tolerance. Proc Natl Acad Sci USA 103:18008–18013
Lewis MJ, Pelham HR (1996) SNARE-mediated retrograde traffic from the Golgi complex to the endoplasmic reticulum. Cell 85:205–215
Lewis MJ, Rayner JC, Pelham HR (1997) A novel SNARE complex implicated in vesicle fusion with the endoplasmic reticulum. EMBO J 16:3017–3024
Leyman B, Geelen D, Quintero FJ, Blatt M (1999) A tobacco syntaxin with a role in hormonal control of guard cell ion channels. Science 283:537–540
Li L, Shimada T, Takahashi H, Ueda H, Fukao Y, Kondo M, Nishimura M, Hara-Nishimura I (2006) MAIGO2 is involved in exit of seed storage proteins from the endoplasmic reticulum in Arabidopsis thaliana. Plant Cell 18:3535–3547
Nakajima K, Hirose H, Taniguchi M, Kurashina H, Arasaki K, Nagahama M, Tani K, Yamamoto A, Tagaya M (2004) Involvement of BNIP1 in apoptosis and endoplasmic reticulum membrane fusion. EMBO J 23:3216–3226
Perry RJ, Mast FD, Rachubinski RA (2009) Endoplasmic reticulum-associated secretory proteins Sec20p, Sec39p, and Dsl1p are involved in peroxisome biogenesis. Eukaryot Cell 8:830–843
Reilly BA, Kraynack BA, VanRheenen SM, Waters MG (2001) Golgi-to-endoplasmic reticulum (ER) retrograde traffic in yeast requires Dsl1p, a component of the ER target site that interacts with a COPI coat subunit. Mol Biol Cell 12:3783–3796
Ren Y, Yip CK, Tripathi A, Huie D, Jeffrey PD, Walz T, Hughson FM (2009) A structure-based mechanism for vesicle capture by the multisubunit tethering complex Dsl1. Cell 139:1119–1129
Sanderfoot AA, Assaad FF, Raikhel NV (2000) The Arabidopsis genome. An abundance of soluble N-ethylmaleimide sensitive factor adaptor protein receptors. Plant Physiol 124:1558–1569
Schmitt HD (2010) Dsl1p/Zw10: common mechanisms behind tethering vesicles and microtubules. Trends Cell Biol 20:257–268
Surpin M, Raikhel NV (2004) Traffic jams affect plant development and signal transduction. Nat Rev Mol Cell Biol 5:100–109
Sweet DJ, Pelham HR (1992) The Saccharomyces cerevisiae SEC20 gene encodes a membrane glycoprotein which is sorted by the HDEL retrieval system. EMBO J 11:423–432
Sweet DJ, Pelham HR (1993) The TIP1 gene of Saccharomyces cerevisiae encodes an 80 kDa cytoplasmic protein that interacts with the cytoplasmic domain of Sec20p. EMBO J 12:2831–2840
Sztul E, Lupashin V (2006) Role of tethering factors in secretory membrane traffic. Am J Physiol Cell Physiol 290:C11–C26
Tamura K, Shimada T, Ono E, Tanaka Y, Nagatani A, Higashi SI, Watanabe M, Nishimura M, Hara-Nishimura I (2003) Why green fluorescent fusion proteins have not been observed in the vacuoles of higher plants. Plant J 35:545–555
Tripathi A, Ren Y, Jeffrey PD, Hughson FM (2009) Structural characterization of Tip20p and Dsl1p, subunits of the Dsl1p vesicle tethering complex. Nat Struct Mol Biol 16:114–123
Uemura T, Ueda T, Ohniwa RL, Nakano A, Takeyasu K, Sato MH (2004) Systematic analysis of SNARE molecules in Arabidopsis: dissection of the post-Golgi network in plant cells. Cell Struct Funct 29:49–65
Uemura T, Sato T, Aoki T, Yamamoto A, Okada T, Hirai R, Harada R, Mori K, Tagaya M, Harada A (2009) p31 deficiency influences endoplasmic reticulum tubular morphology and cell survival. Mol Cell Biol 29:1869–1881
Vicient CM, Delseny M (1999) Isolation of total RNA from Arabidopsis thaliana seeds. Anal Biochem 268:412–413
Wang LC, Tsai MC, Chang KY, Fan YS, Yeh CH, Wu SJ (2011) Involvement of the Arabidopsis HIT1/AtVPS53 tethering protein homologue in the acclimation of the plasma membrane to heat stress. J Exp Bot 62:3609–3620
Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2:1565–1572
Zhao P, Liu F, Ma M, Gong J, Wang Q, Jia P, Zheng GC, Liu H (2011) Overexpression of AtLEA3-3 confers resistance to cold stress to Escherichia coli and provides enhanced osmotic stress tolerance and ABA sensitivity in Arabidopsis thaliana. Mol Biol (Mosk) 45:851–862
Zhao P, Liu F, Zhang B, Liu X, Wang B, Gong J, Yu G, Ma M, Lu Y, Sun J, Wang Z, Jia P, Liu H (2012) MAIGO2 is involved in abscisic acid-mediated response to abiotic stresses and Golgi-to-ER retrograde transport. Physiol Plant. doi:10.1111/j.1399-3054.2012.01704.x
Zhou ZY, Zhang CG, Wu L, Zhang CG, Chai J, Wang M, Jha A, Jia PF, Cui SJ, Yang M, Chen R, Guo GQ (2011) Functional characterization of the CKRC1/TAA1 gene and dissection of hormonal actions in the Arabidopsis root. Plant J 66:516–527
Zhu JH, Gong ZZ, Zhang CQ, Song CP, Damsz B, Inan G, Koiwa H, Zhu JK, Hasegawa PM, Bressan RA (2002) OSM1/SYP61: a syntaxin protein in Arabidopsis controls abscisic acid-mediated and non-abscisic acid-mediated responses to abiotic stress. Plant Cell 14:3009–3028
Acknowledgments
We thank Dr. Ian Moore (University of Oxford) for providing plasmid pVKHEn6-GFP-HDEL; Dr. Ikuko Hara-Nishimura (Kyoto University) for providing sp-GFP-2SC/pBI221, sp-GFP-CTPP/pBI221, sp-NTPP-GFP/pBI221 plasmids and transgenic seeds sp-GFP-2SC/pBI121, and Dr. Zhaoyang Zhou and Qianqian Qin for providing ethylene (Zhou et al. 2011) and GA signal homozygous mutants. This work was supported by National Natural Science Foundation of China (No. 30771091, 30970234, and 41201048) and the Program for New Century Excellent Talents in University (NCET-11-0212) by the Ministry of Education to H. L. (2011), by Grant 29Y127E71 from the “One Hundred Talents” Project of the Chinese Academy of Sciences, and by the Foundation for Excellent Youth Scholars of CAREERI, CAS (51Y251A11).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by J.-H. Liu.
P. Zhao and F. Liu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
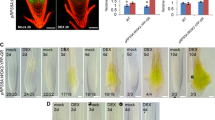
Fig. S1 Phenotypes of the wt and sec20 plants with or without treatments at 7 day after stratification. Photographs were taken at the end of treatments.
Fig. S2 Expression patterns of AtSec20 in the ABA, GA, and ethylene signal mutants. RNA was isolate from 7 d-old plants. Transcript levels were measured by real-time RT-PCR, and actin2 was used as an internal control. The expression levels in Col 0 and Ler were designated as a value 1. Data represent the mean ± SD. Bars with different letters were significantly different at the 0.01 level.
Rights and permissions
About this article
Cite this article
Zhao, P., Liu, F., Wang, B. et al. AtSec20 is involved in osmotic stress tolerance and AtSec20 mutation unaffects the integrity of intracellular organelles and the anterograde biosynthetic trafficking. Acta Physiol Plant 35, 1625–1632 (2013). https://doi.org/10.1007/s11738-012-1205-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1205-6