Abstract
Key message
Interactor of WOX2, CDC48A, is crucial for early embryo patterning and shoot meristem stem cell initiation, but is not required for WOX2 protein turnover or subcellular localization.
Abstract
During Arabidopsis embryo patterning, the WUSCHEL HOMEOBOX 2 (WOX2) transcription factor is a major regulator of protoderm and shoot stem cell initiation. Loss of WOX2 function results in aberrant protodermal cell divisions and, redundantly with its paralogs WOX1, WOX3, and WOX5, compromised shoot meristem formation. To elucidate the molecular basis for WOX2 function, we searched for protein interactors by IP–MS/MS from WOX2-overexpression roots displaying reprogramming toward shoot-like cell fates. Here, we report that WOX2 directly interacts with the type II AAA ATPase molecular chaperone CELL DIVISION CYCLE 48A (CDC48A). We confirmed this interaction with bimolecular fluorescence complementation and co-immunoprecipitation and found that both proteins co-localize in the nucleus. We show that CDC48A loss of function results in protoderm and shoot meristem stem cell initiation defects similar to WOX2 loss of function. We also provide evidence that CDC48A promotes WOX2 activity independently of proteolysis or the regulation of nuclear localization, common mechanisms of CDC48A function in other processes. Our results point to a new role of CDC48A in potentiating WOX2 function during early embryo patterning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The basic body plan of the embryo in Arabidopsis thaliana (hereafter Arabidopsis) is established during early embryogenesis. At the eight-cell stage, four groups of cells have formed with different developmental fates along the apical–basal embryo axis: the apical domain gives rise to the shoot meristem and cotyledons, the central domain to the hypocotyl and root, the hypophyseal cell to the distal root meristem, and the suspensor connects the embryo to the maternal tissue. At the 16-cell stage, the protoderm is separated from the inner cells by a round of periclinal cell divisions, setting up the radial (inner–outer) embryo axis. During subsequent developmental stages, cells acquire their developmental fate based on their spatial position. At the shoot and root poles, the primordia of the apical meristems are formed, which will give rise to the cells required for continuous plant growth after germination (Bäurle and Laux 2003; Capron et al. 2009; Laux et al. 2004; Yoshida et al. 2014).
WOX2 belongs to the youngest evolutionary clade of the WUSCHEL RELATED HOMEOBOX (WOX) family of transcription factors, many of which regulate stem cell and embryo development in angiosperms (Haecker et al. 2004; van der Graaff et al. 2009). Expression of the WOX2 gene commences in the zygote, is successively confined to the apical protoderm of the embryo, and terminates just before the shoot meristem becomes apparent (Haecker et al. 2004). wox2 loss-of-function embryos display patterning defects at different stages. Among these defects are abnormal anticlinal cell divisions at the eight-cell stage, resulting in incomplete protoderm formation. From the globular stage onwards, WOX2 promotes the formation of a cytokinin domain in the center of the apical embryo and is required for the timely initiation of the shoot meristem and expression of the stem cell marker CLAVATA3 (CLV3) (Zhang et al. 2017). These defects are enhanced in the wox1-2 wox2-1 wox3-2 wox5-1 (wox1235) quadruple mutant (Breuninger et al. 2008). However, genetic analyses indicate that WOX2 is a major regulator in embryo patterning, whereas WOX1, WOX3, and WOX5 seem to take over redundant functions only if WOX2 is non-functional.
Once initiated, the maintenance of the shoot meristem is governed by the WOX2 paralog and founding member of the WOX family, the WUSCHEL (WUS) gene. WUS is expressed in the organizing center (OC) of the shoot meristem from where it maintains the overlying stem cell pool and directly promotes the expression of the negative feedback signal CLV3 (Lenhard and Laux 2003; Schoof et al. 2000; Yadav et al. 2011). In the embryo, WUS expression commences in the precursor cells of the OC at the 16-cell stage. Still, it is neither sufficient nor required for stem cell initiation, as indicated by the timely onset of CLV3 expression at the heart stage of embryogenesis (Mayer et al. 1998; Tucker et al. 2008; Zhang et al. 2017).
It is unclear why WOX2 can initiate shoot meristem formation but not WUS, although WUS is already expressed in the precursor cells of the OC, and WOX2 can partially substitute for WUS in postembryonic stem cell maintenance (Dolzblasz et al. 2016). Both proteins contain a conserved homeodomain and a WUS-box, but WUS also contains acidic and EAR domains not found in WOX2 (van der Graaff et al. 2009), suggesting, at least in part, different molecular workings of both proteins.
CELL DIVISION CYCLE 48 (CDC48) (with homologs CDC48p in yeast and p97/vasolin-containing protein in mammals) has been found in many organisms to be involved in various cellular processes, including cellular signaling, protein degradation, vesicular trafficking, and autophagy (Baek et al. 2013; Begue et al. 2017). The active form of CDC48 is a barrel-shaped homohexameric complex using energy from ATP hydrolysis to modify proteins. CDC48 can function as a ubiquitin-dependent unfoldase/segregase and requires interaction with diverse cofactors for substrate selection (Bodnar and Rapoport 2017; Meyer et al. 2012; Ramadan et al. 2017). Although homologs of CDC48 have been identified in a few plant phylogenetic clades (Begue et al. 2017), functional studies of CDC48 have been majorly conducted in Arabidopsis, where five CDC48 paralogs exist: A (AT3G09840), B (AT3G53230), C (AT5G03340), D (AT2G03670), and E (AT3G01610) (Copeland et al. 2016), all of which are expressed in the embryo (Hofmann et al. 2019). While most studies have been carried out on CDC48A, very few have identified the functions of the other paralogs in Arabidopsis, albeit in other contexts (Niehl et al. 2012; Yang et al. 2022).
We searched for interacting proteins to gain insight into the molecular mechanism by which WOX2 functions in early patterning. Here, we report that CDC48A is a direct interactor of WOX2 and is required for protoderm formation and shoot meristem development. Our results further suggest that CDC48A potentiates WOX2 activity during embryo patterning, independently of its canonical functions in protein degradation or nuclear localization of client proteins, suggesting a previously unreported function of CDC48A.
Results
WOX2 interacts with the AAA+ ATPase CDC48A in planta
To investigate the molecular mechanism of WOX2 function in early embryo patterning and stem cell initiation (Zhang et al. 2017), we searched for proteins interacting with WOX2 by IP–MS/MS. Because obtaining sufficient protein from early-stage embryos is difficult, we asked whether ectopically expressing WOX2 in root cells would induce shoot meristem stem cell identity, as previously reported for its close paralog WUS (Gallois et al. 2004) and thus enable us to extract WOX2 protein interactors from reprogrammed root cells as a proxy. To circumvent potential detrimental effects by constitutively overexpressing WOX2, we generated plants with WOX2-YFP fused to a glucocorticoid receptor (GR) (Aoyama and Chua 1997) driven by the ubiquitous RIBOSOMAL PROTEIN SUBUNIT 5A (RPS5A) promoter (Weijers et al. 2001). Upon treating pRPS5A:WOX2-YFP-GR roots with 10-μM DEX for 2 h, we found that WOX2-YFP-GR completely shifts from the cytoplasm into nuclei of the root cells (Fig. 1a, b). 2 days after DEX induction, the roots of 6-day-old seedlings expanded in width and generated more root hairs. 3–4 days of DEX treatment resulted in the roots greening at the meristematic region and bulges protruding from the root surface. After 5–10 days of DEX treatment, the bulges were also present in the transition and differentiation zones. The bulges did not develop into lateral roots but into a central dome surrounded by several primordia-like protrusions reminiscent of a shoot meristem surrounded by leaf primordia (Fig. 1c). Furthermore, a qRT-PCR showed that a 2-day incubation with DEX caused upregulated expression of the shoot stem cell marker CLV3 (Fig. 1e, Table S1) and the WOX2 effector gene in shoot meristem initiation, PHABULOSA (PHB) (Fig. 1f, Table S1). In contrast, wild-type roots showed no phenotypic abnormalities or altered relative expression levels of CLV3 and PHB after treatment with DEX (Fig. 1d–f, Table S1). These observations suggest that ectopic WOX2 activity reprograms root cells toward at least some aspects of shoot meristem formation. Therefore, we used the roots of DEX-induced pRPS5A:WOX2-YFP-GR as a proxy for WOX2 transcriptional activity in shoot meristem initiation and searched for interactors. Since, at this time, WOX2-YFP-GR has moved into the nucleus, we harvested roots after a 4-h DEX induction, followed by an IP–MS/MS using an anti-GFP antibody. We hypothesized that this strategy would preferentially identify proteins interacting with WOX2 in the nucleus, where it plausibly executes its regulatory function. From several enriched proteins (Table S2), we focused on CDC48A for further analysis because cdc48a T-DNA insertion mutants have been reported to display embryo development defects (Park et al. 2008). We confirmed the interaction between CDC48A and WOX2 by bimolecular fluorescence complementation (BiFC) in Arabidopsis mesophyll protoplasts (Fig. 2a) and by co-immunoprecipitation from Arabidopsis plants stably expressing WOX2-YFP-GR and CDC48-FLAG proteins (Fig. 2b, c). Furthermore, by analyzing different truncated variants, we showed that the N-terminal domain of CDC48A is required for interaction with WOX2 (Fig. S1a, b), consistent with previous reports showing that the N-terminal domain of CDC48 is responsible for binding to cofactors and substrates (Park et al. 2007).
Ectopic WOX2 expression in the root leads to over-proliferation and expression of shoot-like traits. a and b Expression of pRPS5A:WOX2-YFP-GR (green) in the wild-type background of roots grown for 5 days on ½MS with 2 h mock treatment (a) or ½MS with 2 h 10 μM DEX treatment (b). Cell walls were stained by propidium iodide (red). n > 15 for each treatment. Scale bars: 50 μm. c and d Root phenotypes of pRPS5A:WOX2-YFP-GR (c) and wild-type (d) seedlings treated with either mock or 10 μM DEX from 2 to 10 days as indicated. Scale bars: 100 μm. e and f Relative expression levels of CLV3 (e) and PHB (f) from the roots of 6-day-old (4 days on MS with 2 days on 10 μM DEX or mock treatment) wild type (WT) and pRPS5A:WOX2-YFP-GR. Data represents mean values ± SD of three biological replicates by quantitative RT-PCR normalized to the internal control (TIP41). a and b indicate significance categories (p < 0.001) by one-way ANOVA with a post hoc Tukey–Kramer test
CDC48A interacts with WOX2 in plantabb. a Confocal microscopy images of a BiFC assay showing interaction between WOX2 and CDC48A proteins in Arabidopsis thaliana leaf mesophyll protoplasts. The negative control shows no interaction between WOX2 and EID1. As a positive control, EID1 and ASK1 showed interaction with each other. The percentages of protoplasts with YFP signal and the numbers of total protoplasts observed are indicated. Scale bars: 20 μm. b and c Co-IP results from wild type, pRPS5a:WOX2-YFP-GR, p35S:CDC48A-FLAG and p35S:CDC48A-FLAG/ pRPS5a:WOX2-YFP-GR. Proteins extracted from 5-day-old seedlings after a 4-h 10 μM DEX induction were immunoprecipitated by anti-GFP beads (b) or anti-FLAG beads (c). Western blots using anti-GFP and anti-FLAG to detect WOX2 and CDC48A, respectively, are shown. Arrowheads indicate CDC48A-FLAG, and arrows indicate WOX2-YFP-GR. Coomassie blue staining as loading controls are shown
CDC48A knockout mutants are defective in embryonic protoderm and shoot meristem formation, similar to the WOX2 loss-of-function mutants
Previous reports have shown that wox2 mutant embryos fail to establish a contiguous protoderm at the 16-cell stage due to abnormal cell divisions (Breuninger et al. 2008) and to initiate shoot meristem stem cells at the early heart stage, and these defects are strongly enhanced in the wox1-2 wox2-4 wox3-2 wox5-1 (wox1235) quadruple mutant (Zhang et al. 2017). To address whether the loss of CDC48A function causes similar defects, we studied three T-DNA insertion mutant lines, cdc48a-1, cdc48a-2, and cdc48a-3, where the T-DNA is inserted in the first intron, third exon, and third intron of CDC48A, respectively (Park et al. 2008). All three T-DNA insertion sites are upstream of the sequence encoding the two ATPase domains, and all three segregating T-DNA insertion mutants exhibited the same frequency of embryos with protoderm defects at the 16-cell stage (Fig. S2a–d). This strongly suggests that the observed defects were due to a lack of CDC48A function. We focused on cdc48a-1 for a detailed phenotypic analysis.
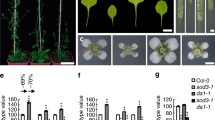
Unlike the wild-type embryos that undergo a round of periclinal cell divisions at the eight-cell stage that separates the outer protodermal layer from the inner cells (Fig. 3a, d), 19% (n = 138) of the segregating embryos from a cdc48a-1/+ mother do not display anticlinal divisions and, consequently, display a disrupted apical protodermal layer (Fig. 3b, d). Notably, these defects are very similar to those in the wox1235 mutant (Fig. 3c, d). Thus, CDC48A and WOX2 appear to play similar roles in embryonic protoderm formation.
CDC48A loss of function shows defects in protoderm formation. a–c Phenotypes of 16-cell stage embryos from wild-type (a), wox1235 (b), and cdc48a-1/+ (c) mother plants. White arrowheads point to the abnormal cell division plane. Scale bar: 10 μm. d Frequencies of cell division patterning phenotypes from 16-cell stage embryos from wild-type (WT), cdc48a-1/+, and wox1235 mother plants. a, b, and c indicate significance categories (p < 0.0001) by Fisher’s exact test with Bonferroni correction
We next investigated shoot meristem initiation, monitoring the expression of the stem cell reporter gene pCLV3:er-tdTomato (Zhang et al. 2017). We detected pCLV3:er-tdTomato in 100% wild-type embryos at the heart stage (Fig. 4a, g), the late heart-to-torpedo stages (Fig. 4c, g), and the bent-cotyledon stage (Fig. 4e, g). By contrast, an increasing fraction of the segregating cdc48a-1/+ embryos lacked CLV3 reporter expression during progressive development: 6.4% at the heart stage (Fig. 4b, g), 4.8% at late heart-torpedo stages (Fig. 4d, g), and 20% at the bent-cotyledon stage (Fig. 4f, g). Furthermore, pCLV3:er-tdTomato expression levels were weaker than in the wild type in the remaining embryos (Fig. S3). These observations suggest that CDC48A is required for the timely initiation and normal levels of embryonic CLV3 expression.
CDC48A is necessary for shoot meristem development. a–f pCLV3:er-tdTomato expression in heart (a, b), torpedo (c and d), and bent-cotyledon (e, f) stage embryos from mother plants of the indicated genotypes. Scale bars: 20 μm. g Frequencies of embryos with and without pCLV3:er-tdTomato expression. WT, wild type; n.s. not significant, ****, p < 0.0001 (Fisher’s exact test). h Frequencies of 10-day-old seedlings with or without a SAM. WT, wild type. a, b, and c indicate significance categories (p < 0.01) by Fisher’s exact test with Bonferroni correction. i–n Representative images of 10-day-old seedling phenotypes of the indicated genotypes. The functional shoot apical meristem (SAM) is present in the wild type (i) and cdc48a-1/+ (k). Terminated SAM in cdc48a-1/- (j). wox1235 shows wild type-like shoot with a functional SAM (l), or a single cotyledon (c) with a SAM and one rosette leaf (rl) (m), or a pin-like shoot structure (n). Scale bars: 2 mm
We then examined the phenotypes of 10-day-old seedlings from a cdc48a-1/+ mother plant and PCR-genotyped 10.3% of the seedlings (n = 78) as homozygous cdc48a-1, 50% as cdc48a-1/+, and 32.0% as wild type, with the remaining 7.7% of the seeds not germinating (Table S3). The homozygous cdc48a-1 seedlings displayed a terminated shoot apical meristem without producing any leaves (Fig. 4h, j), a short root, and failed to grow further after (data not shown). The heterozygous cdc48a-1/+ seedlings showed normal root and vegetative shoot growth (Fig. 4h, k). In comparison, around 26% (n = 112) of 10-day-old wox1235 seedlings exhibited no visible shoot meristem (Fig. 4h, m, and n), consistent with previous findings (Zhang et al. 2017).
Because the cdc48a-1/+ protodermal defects, the delay or absence of CLV3 expression, and the absence of a functional shoot meristem are very similar to the defects exhibited by wox2-1 (Breuninger et al. 2008) and wox1235 (Zhang et al. 2017), we addressed the genetic relationship of CDC48A and WOX2. Since a wox1235 cdc48a-1/+ plant could not be constructed due to close genetic linkage between the WOX5 and CDC48A loci (Table S4), we analyzed the wox2-2 cdc48a-1/+ double mutant. We found that the frequency of aberrant protoderm formation at the 16-cell embryo stage was not significantly enhanced compared to wox2-2 or cdc48a-1/+ embryos (Figs. 5a–d and f). Likewise, we did not find any enhanced shoot apical meristem defects in segregating wox2-2 cdc48a-1/+ seedlings compared to cdc48a-1/+ or wox2-2 (Table S5, Fig. 5g–q). These findings support the hypothesis that CDC48A and WOX2 genetically act in one pathway in protoderm and shoot meristem formation.
CDC48A and WOX2 function in the same process. a–e 16-cell stage embryos from mother plants of the indicated genotypes. White arrowheads point to abnormal cell division planes. FM4-64 counterstaining of the plasma membranes is shown (magenta). Scale bar: 10 μm. f Frequencies of embryo phenotypes at the 16-cell embryo stage of the indicated genotypes. WT, wild type. a and b indicate significance categories (p < 0.001) by Fisher’s exact test with Bonferroni correction. g–p Representative images of 10-day-old shoot phenotypes of the wild type (G), cdc48a-1/+ (h–i), wox2-2 (j–l), wox2-2 cdc48a-1/+ (m–p). Shoot apical meristem (SAM) absence is observed in cdc48a-1/+ (i) and wox2-2 cdc48a-1/+ (p). Fused cotyledons are observed in wox2-2 (k) and wox2-2 cdc48a-1/+ (n). Single cotyledon (c) with one rosette leaf (rl) is seen in wox2-2 (l) and wox2-2 cdc48a-1/+ (o). Scale bars: 1 mm. q Frequencies of 10-day-old seedling shoot phenotypes in the indicated genotypes. WT, wild type. a and b indicate significance categories concerning SAM presence (p < 0.0001) by Fisher’s exact test with Bonferroni correction. c and d indicate significance categories concerning cotyledon outgrowth (p < 0.01) by Fisher’s exact test with Bonferroni correction. SAM shoot apical meristem, DAG days after germination
We then asked whether increased WOX2 activity could compensate for the loss of CDC48A by introducing a second copy of WOX2. Indeed, expressing pWOX2(6.5 kb):GFP-WOX2 (hereafter gWOX2-GFP) fully rescued the protoderm defect in cdc48a-1/+ 16-cell stage embryos (Fig. 5e, f) and globular stage embryos (Fig. S4d, e). This suggests that CDC48A potentiates the function of physiological levels of WOX2 in protoderm formation. By contrast, gWOX2-GFP did not rescue the shoot meristem defects in segregating 10-day-old cdc48a-1/+ seedlings (Table S5, Fig. 5q).
In addition to their shared phenotypes, we found several aberrations in the cdc48a-1 mutant not observed in wox2 or wox1235. First, around 15% of the embryos in a segregating cdc48a-1 population that initiated pCLV3:er-tdTomato expression failed to maintain it (Fig. 4g). This suggests that CDC48A not only plays a role in shoot stem cell initiation, but also in shoot stem cell maintenance. Second, we found a delay in embryo development in a fraction of the segregating cdc48a-1/+ (Table S6). In the sixth silique, the majority (43.5%, n = 147) of the embryos are at the torpedo stage in a wild-type plant. By contrast, only 0.5% (n = 184) of the embryos from a cdc48a-1/+ mother plant are at the torpedo stage, whereas the remaining embryos are at the globular to heart stages (Table S6). In the 15th silique, all embryos of a wild-type mother were fully green at the bent-cotyledon stage (Fig. 6a, d, Table S6). By contrast, only a smaller fraction (89.3%, n = 150) of embryos from the 15th silique of a cdc48a-1/+ mother was at the bent-cotyledon stage, and remaining ovules were pale with embryos at the heart-to-torpedo stages (Fig. 6b–d, Table S6). Third, although the frequencies of cdc48a-1 seedlings without an apparent shoot meristem varied (Tables S3 and S5), we never observed 25% as expected for segregating homozygotes. Furthermore, we did not observe a significant number of lethal embryos in cdc48a-1/+ siliques (Table S3). Therefore, we performed reciprocal crosses and found a reduced paternal transmission of the mutant allele (Table S7). This can explain the underrepresentation of segregating cdc48a-1 homozygotes, consistent with previous observations (Park et al. 2008).
CDC48A loss of function shows defects in timely embryo development. a, b Developing seeds of wild type (a) and cdc48a-1/+ (b) in the 15th silique. Pale seeds are indicated by white arrowheads. c Pale seeds from the 15th silique contained heart-to-torpedo stage embryos. Scale bar: 20 μm. d Frequencies of developing seed phenotypes from wild-type (WT) and cdc48a-1/+ 15th siliques. ***, p < 0.001 (Fisher’s exact test)
Thus, in addition to its common functions with WOX2, CDC48A plays a role in other processes.
CDC48A co-localizes with WOX2 in embryo nuclei
To examine whether CDC48A and WOX2 expression patterns overlap during embryogenesis, we analyzed transgenic plants expressing a YFP-CDC48A fusion protein from its native promoter comprising 754 bp upstream of its transcription start site (Park et al. 2008). pCDC48A:YFP-CDC48A fully complements the protodermal cell division patterning defect of the segregating cdc48a-1/+ embryos and the shoot apical meristem defect of homozygous cdc48a-1 seedlings (Fig. S5a, b), indicating that the transgene is functional in these processes. We detected pCDC48A:YFP-CDC48A expression from the four-cell stage in every cell until the globular embryo stage. At the early heart stage, pCDC48A:YFP-CDC48A expression becomes restricted to the protodermal cell layer (Fig. 7a). Similarly, expression of the functional pWOX2(6.5 kb):GFP-WOX2 reporter (Fig. S6) is detected in all cells of the embryo proper until the early heart stage and is then restricted to the protoderm (Fig. 7b). We confirmed their overlapping expression patterns in embryos co-expressing pCDC48A:YFP-CDC48A with a functional pWOX2(3.5 kb):tdTomato-WOX2 (Fig. 7c, Fig. S5).
CDC48A co-localizes with WOX2 in the nucleus. a–c Representative confocal microscopy images of pCDC48A:YFP-CDC48A expression (a) and pWOX2(6.5 kb):GFP-WOX2 expression (b) at the indicated embryo stages. n > 30 per developmental stage. Scale bars: 20 μm. c Co-expression of pCDC48A:YFP-CDC48A and pWOX2(3.5 kb):tdTomato-WOX2 at the globular embryo stage. n > 30. Scale bar: 20 μm. d and e Confocal microscopy images showing co-localization of 35S:tdTomato-CDC48A and 35S:NTF (nuclear targeting GFP fusion protein) (d), and co-localization of 35S:tdTomato-WOX2 and 35S:GFP-CDC48A (e) in Arabidopsis protoplasts. (n > 30). Scale bars: 20 μm. f and g Confocal microscopy images showing co-localization of 35S:GFP-CDC48A and 35S:tdTomato-WOX2 (f), and 35S:GFP-CDC48A and 35S:ER-mCherry (g) in tobacco leaf cells. (n > 10). Scale bars: 20 μm
To address whether and where the intracellular localization of CDC48A and WOX2 proteins overlap as expected for interacting proteins, we co-transformed 35S:GFP-CDC48A and 35:tdTomato-WOX2 into Arabidopsis leaf mesophyll protoplasts and Nicotiana benthamiana leaf cells. We found both proteins co-localized in the nucleus in both species (Fig. 7d–g). In addition, GFP-CDC48A was also present in the cytoplasm in both assays (Fig. 7d–g).
Thus, pCDC48A:YFP-CDC48A and pWOX2(6.5 kb):GFP-WOX2 expression patterns are strikingly similar during early embryogenesis, and their intracellular localizations overlap in the nucleus, consistent with their putative interaction.
CDC48A does not affect WOX2 protein stability or nuclear accumulation in the embryo
Considering its established canonical function, we then asked how CDC48A might affect WOX2 function. First, a CDC48 function in targeting client proteins toward degradation has been reported in many studies (Begue et al. 2017). However, we found that the levels of WOX2-YFP-GR protein were indistinguishable among cdc48a-1/+, CDC48A overexpressor, and wild-type roots (Fig. S7), indicating that CDC48A is not required for ectopically-expressed WOX2’s stability. Second, since previous studies also reported a role of CDC48 in the nuclear localization of client proteins (Ndoja et al. 2014; Wilcox and Laney 2009), we considered whether CDC48A affects the subcellular localization of WOX2. To this end, we compared the subcellular distributions of gWOX2-GFP between wild-type and segregating cdc48a-1 embryos. Because the first protoderm developmental defect occurs at the 16-cell stage, we focused on the 8-cell, 16-cell, and early globular stages. In all three developmental stages, we observed neither a change in nuclear to cytoplasmic GFP signal ratio nor a change in total GFP content in both the nucleus and the cytoplasm between the wild-type and cdc48a-1/+ backgrounds (Fig. S8a–c). These results demonstrate that CDC48A is not a limiting factor for the nuclear localization and stability of the WOX2 protein in the embryo.
Discussion
WOX2 is a central regulator for protoderm and shoot meristem initiation during early embryo patterning (Zhang et al. 2017). However, the molecular mechanisms of its function have remained enigmatic. Here, we isolated CDC48A as a putative interactor of WOX2 and showed that it is required for protoderm and shoot meristem initiation similar to WOX2. In the following, we will address the role of CDC48A and its implications for WOX2 function in embryo patterning.
WOX2 expression in the root results in shoot-like reprogramming
Since the cells expressing WOX2 in the embryo are too scarce, we have chosen the overexpression of WOX2 in the root as a proxy for its function in shoot meristem initiation to search for interactors by IP–MS/MS. Several observations support this choice: WOX2 overexpression in the root induced the formation of shoot meristem-like structures and increased expression of two WOX2 downstream genes, the stem cell marker gene CLV3 and PHB, which mediates WOX2 function in embryonic shoot meristem initiation (Zhang et al. 2017). A similar conversion of root to shoot cell fates has been previously reported for the WUS gene (Gallois et al. 2004; Negin et al. 2017), the major regulator of stem cell maintenance. Furthermore, WOX2 can replace WUS in postembryonic shoot meristem maintenance, supporting similar protein functions (Dolzblasz et al. 2016). Although broadly overexpressed in our experiment, the discrete appearance of proliferating bulges rather than a complete conversion of all root cells and the relatively modest increase of CLV3 and PHB expression suggest that WOX2 induces stable shoot meristem features only at restricted foci. A plausible interpretation is that initial differences between cells and/or confinement of cell fates by lateral inhibition limit the formation of novel structures to a small region within the WOX2-expressing cells. Similar observations have been made for the induction of ectopic shoot meristem when ubiquitously expressing WUS (Lu et al. 2023; Zuo et al. 2002).
WOX2 and CDC48A are required for protoderm and shoot meristem initiation
The expression patterns of WOX2 and CDC48A proteins overlap during early embryo patterning, cdc48a-1/+, and wox2-2 embryos display strikingly similar defects in protoderm division patterns, and cdc48a-1/+ wox2-2 double mutants display non-additive effects at this stage. Together with the delayed expression onset of the shoot meristem stem cell marker CLV3 during embryogenesis and a lack of a functional shoot meristem at the seedling stage, these findings suggest that WOX2 and CDC48A act together during protoderm formation and shoot meristem initiation.
That an extra copy of WOX2 compensates for the absence of CDC48A activity, specifically in protoderm formation, is curious. One plausible explanation is that CDC48A potentiates physiological levels of WOX2 function in the wild type, the absence of which can be overcome by a surplus of WOX2 protein. A role of CDC48A in regulating Arabidopsis cell divisions is also suggested by previous reports. Park et al. (2008) found that cdc48a-1 primary roots post-germination exhibited abnormally orientated cell division planes in the meristematic region (Park et al. 2008). Furthermore, during cytokinesis in Arabidopsis, CDC48A has been shown to interact with the member of the SNARE protein complex, the syntaxin 5 ortholog SYP31, possibly mediating membrane fusion and/or disassembly at the cell division plane (Rancour et al. 2002). Finally, Arabidopsis CDC48A has been found to promote mitotic spindle assembly and cell division in yeast cells (Feiler et al. 1995). While these findings support a role of CDC48 during cytokinesis, understanding how its interaction with WOX2 influences protodermal cell division patterning requires further studies.
One obvious question is whether the defects in shoot meristem stem cell initiation of wox2 and cdc48a-1 mutants resulted from the previous failure to separate the protodermal layer correctly or whether these events reflect separate functions of WOX2/CDC48A. Whereas the severe pleiotropic defects make addressing this question in the cdc48a-1 mutant difficult, two observations argue against this possibility. First, our previous analysis showed that protodermal defects in genetic combinations of the wox2 mutant are more frequent than shoot meristem defects (Breuninger et al. 2008). Furthermore, we report that an extra WOX2 copy can complement the protodermal of the cdc48a-1 mutant but not its shoot meristem defects. What could the downstream processes be? While the targets of WOX2 in protoderm formation have yet to be revealed, it is tempting to speculate that the WOX2/CDC48A interaction may mediate the downregulation of MIR166B expression in the apical protoderm and the subsequent activation of HD-ZIP III transcription factors and, ultimately, the cytokinin to auxin ratio during shoot stem cell initiation, as reported for WOX2 (Zhang et al. 2017). Since the pleiotropic effects of cdc48a-1/+ seedlings hamper a more detailed comparison with the wox2 or wox1235 seedlings, further work is necessary to unveil the molecular mechanisms of WOX2/CDC48A function.
How common is the function of CDC48A in protoderm formation and shoot meristem initiation in the plant kingdom? In Nicotiana glutinosa, the NgCDC48 homolog was found to be crucial for normal vegetative and reproductive shoot development, with its loss of function resulting in seedling lethality (Bae et al. 2009). However, the underlying mechanisms and a potential connection to a WOX2 homolog in this species are unknown. Functional studies of CDC48 homologs in other plant species are currently lacking.
WOX2 and CDC48A proteins interact
We found that WOX2 and CDC48A proteins interact by different experiments: IP–MS/MS, BiFC in transiently transformed Arabidopsis cells, and Co-IP in stable Arabidopsis lines. We tested two hypotheses for well-known CDC48A functions on WOX2: protein degradation and nuclear localization. First, many studies show that CDC48A can segregate client proteins toward proteasomal degradation, for example, by promoting centromere disassembly during the cell cycle (Merai et al. 2014). However, our results suggest that this is unlikely for WOX2. wox2-2 (and the enhanced wox1235 quadruple mutant) and cdc48a-1/+ display similar defects in protoderm and shoot meristem development, not opposite ones as one would expect if CDC48A promoted WOX2 proteolysis. Nevertheless, it is also conceivable that CDC48A promotes WOX2 function by removing misfolded WOX2 proteins and submitting it to proteolysis, as has been shown for the CDC48A client protein SOMATIC EMBRYOGENESIS RECEPTOR KINASE1 (Aker and de Vries 2008). However, we have not detected any effect of cdc48a-1/+ or CDC48A-overexpressing plants on the signal strength of GFP-tagged WOX2 protein, making a proteolytic-based mechanism unlikely. Second, it has also been reported that CDC48 can mediate the nuclear localization of client transcription factors (Ndoja et al. 2014; Wilcox and Laney 2009). However, we did not detect any changes in the nuclei/cytoplasmic ratio of GFP-tagged WOX2 protein in embryo cells, arguing against such a mechanism during embryo patterning. Further studies on the WOX2–CDC48A interaction are required to pinpoint its precise mechanism influencing embryo patterning.
Materials and methods
Plant materials and growth conditions
The Arabidopsis thaliana accession used in this study was Columbia (Col-0). wox2-2 is a T-DNA insertion line in the Col-0 background described before (Haecker et al. 2004). wox1-2 wox2-4 wox3-2 wox5-1 (wox1235) all have T-DNA insertions in the Col-0 background and were described before (Zhang et al. 2017). cdc48a-1, cdc48a-2, and cdc48a-3 are T-DNA insertion mutants in the Col-0 background previously described (Park et al. 2008). pCDC48A:YFP-CDC48A cdc48a-1/+ was also obtained from Dr. Sookhee Park (Park et al. 2008). The mutants, transgenic lines, plasmids, primers, and genotyping methods used in this study are summarized in Tables S8, S9, S10, S11, and S12.
Arabidopsis and tobacco (Nicotiana benthamiana) seeds were sown on wet soil in pots, stratified at 4 °C for 2 days, and then grown in a phytochamber at 21 °C under long-day conditions (16 h light, 8 h dark). For seeds sown on ½ Murashige and Skoog (MS) plates, seeds were sterilized with 70% ethanol for 1 min and then with 1.2% NaClO for 10 min, followed by a 3-time wash with autoclaved distilled water. Seeds were then sown on ½MS plates, stratified at 4 °C for 2 days, and allowed to grow in a growth chamber (Percival Scientific) at 22 °C under long-day conditions (16 h light, 8 h dark). ½ MS media contained 2.15 g/L Murashige and Skoog Medium (Duchefa), 0.5 g/L MES hydrate with pH of 5.7, adjusted by KOH and 8-10 g/L agar (Plant Agar, Duchefa).
Plasmid construction and plant transformation
DNA fragments were amplified from Arabidopsis genomic DNA by PCR with specific primers (Table S11) by Phusion DNA polymerase (NEB) according to the manufacturer’s instructions. PCR fragments were ligated into the pJET1.2 cloning vector (Thermo Fisher Scientific Inc.) following the manufacturer’s instructions. Ligations of DNA fragments into pGreenII binary vectors following specific restriction enzyme digestions were done using T4 DNA Ligase (NEB). Up to 5 µl ligation products were transformed into 50 µl E.coli XL1 Blue electrocompetent cells. Colony PCRs and DNA sequencing were done to confirm positive clones.
To create pRPS5A:WOX2-YFP-GR, WOX2 CDS was amplified with BamHI flanking sites with primers ERp008/009, and vYFP was amplified with BamHI site GAGA linker-NcoI sites using primers ERp045/046. pRPS5A (Weijers et al. 2001), WOX2 CDS and vYFP were then digested with BamHI digestion and ligated into a pGreenII vector with a rat glucocorticoid receptor (GR) domain, a NOS terminator, and kanamycin resistance for both bacteria and plant.
To create gWOX2-GFP, 6.5 kb upstream, the transcription start site of WOX2 as the promoter was amplified with AscI and HindIII flanking sites by primers oWG164/165. GFP was amplified from pWOX2:H2B-GFP (Zhang et al. 2017) and flanked by HindIII and XmaI-linker using primers oWG168/169. WOX2 open reading frame and 1.7 kb 3ʹUTR were amplified, and XmaI was flanked at both sides by primers oWG166/167. PCR fragments in pJET1.2 were digested with the indicated restriction enzymes and ligated into a pGreen II vector with kanamycin and norflurazon resistance for bacteria and plants, respectively.
For 35S:CDC48A-FLAG, CDC48A CDS with three FLAG tags was amplified with BamHI and NotI flanking sites by primers oWG216/217. PCR fragments in pJET1.2 were digested with the indicated restriction enzymes and ligated into a pGreenII vector carrying a 35S promoter and a 35S terminator before and after the insertion site and kanamycin and norflurazon resistance for bacteria and plant, respectively.
To create pWOX2:tdTomato-WOX2, 3.5 kb upstream, the transcription start site of WOX2 was amplified from pWOX2:H2B-GFP (Zhang et al. 2017) and cloned into a pGreenII vector carrying a NOS terminator by ligation independent cloning (LIC) (De Rybel et al. 2011). The adaptor sequence is GATCCCTAGTTGGAATTGGTTTAAACCCAACTCCATAAGG, containing a PmeI site. The vector was then linearized by SpeI and SalI. tdTomato-GAGA linker was amplified by primers oWG470/471 and WOX2 CDS was amplified by primers oWG472/473. Both PCR fragments were then amplified again into tdTomato-GAGA linker-WOX2 by Gibson assembly (Gibson et al. 2009) and ligated into a pGreenII vector, which has kanamycin and MTX resistance for bacteria and plant, respectively.
For the BiFC assays, the CDS of WOX2 was amplified by primers oWG90/91 with added EcoRI and SacII restriction sites and ligated into pRT-SPYNE, a vector with 35S:nYFP (Walter et al. 2004). The CDS of CDC48A was amplified by primers oWG94/95 and ligated into pRT-SPYCE (Walter et al. 2004), a vector carrying 35S:cYFP. CDC48A ΔN was amplified by oWG186/ oWG95. CDC48A ΔC was amplified by oWG94/ oWG188. CDC48A ΔC + D2 was amplified by oWG94/ oWG190. SacII restriction sites at both ends flanked all mutated CDC48A variants. PCR fragments in the pJET1.2 vector were first digested by SacII and ligated into SacII-linearized pRT-SPYCE with 35S:cYFP.
For the WOX2–CDC48A co-localization assays, 35S:NTF, 35S:tdTomato-WOX2, 35S:GFP-CDC48A, and 35S:tdTomato-CDC48A were generated by assembling the linear fragments following the AQUA cloning procedure (Beyer et al. 2015). For the first three constructs, NTF, tdTomato coding sequence, WOX2 CDS, GFP coding sequence and CDC48A CDS were amplified by primer pairs oWG454/ oWG455, oWG440/ oWG441, oWG442/ oWG443, oWG448/ oWG449, oWG450/ oWG451. tdTomato coding sequence was amplified from pWOX2:LTI6b-tdTomato (Zhang et al. 2017). For 35S:tdTomato-CDC48A, tdTomato coding sequence, and CDC48A CDS were amplified by primers oWG440/452 and oWG453/ oWG451, respectively. After the AQUA cloning procedure, PCR fragments were purified and cloned into linearized vector pRT-SPYNE by NotI/ XbaI digestion (Beyer et al. 2015).
The plasmids generated in this study are listed in Table S10. The binary vectors with the corresponding constructs were first transformed into Agrobacterium tumefaciens strain GV3101 with the pSOUP helper plasmid (Koncz and Schell 1986). Plant transformations in Arabidopsis thaliana were carried out using the floral dip method (Clough and Bent 1998).
Dexamethasone treatment of roots
Plants expressing pRPS5A:WOX2-YFP-GR were grown on ½MS medium for 4 days and then transferred to ½MS supplemented with either 10 μM dexamethasone in H20 (Sigma-Aldrich®, D1756) or 10 μM ethanol in H20 (mock treatment). Seedlings were allowed to grow on the respective agar for a selected amount of time for further analyses. The same treatment was done on control wild-type seeds, where needed.
Gene expression analyses
For RNA extraction, around 100 mg of root tips from 6-day-old pRPS5A:WOX2-YFP-GR seedlings following a 48-h + DEX/mock treatment were taken from one sample and frozen immediately in liquid nitrogen. RNeasy Plant Mini Kit (Qiagen) was used to isolate total mRNA according to the manufacturer’s instructions. Gene expression analyses were determined by qRT-PCR. Reverse mRNA transcription was performed from 1 µg total RNA using Superscript III First-Strand Synthesis Super Mix for qRT-PCR (Invitrogen) following the manufacturer’s instructions. The qRT-PCR reaction was performed using LightCycler 480 SYBR Green I Master (Roche) and a LightCycler 480 (Roche) under the following conditions: 98 °C for 5 min, 40 cycles of 98 °C for 10 s, 60 °C for 20 s, and a final 30-s extension at 72 °C in the cycling stage, 95 °C for 5 s, 60 °C for 20 s, and 97 °C for 1 min in the melt curve stage. Data were analyzed with LightCycler 480 software version 1.5.0.39 (Roche). TIP41 (AT4G34270) was used as an internal reference for qRT-PCR as its expression was experimentally reported to be sufficiently stable for seedlings grown on ½MS with or without DEX (Han et al. 2013). The qRT-PCR primers used in this study are listed in Table S11. The relative expression level of each gene is presented as the normalized RQ, as previously described (Rieu and Powers 2009).
Immunoprecipitation followed by tandem mass spectrometry (IP–MS/MS)
2.5 g of fresh weight root material from 5-day-old pRPS5A:WOX2-YFP-GR seedlings after a 4-h 10 μM Dexamethasone (DEX; Sigma-Aldrich®, D1756) treatment, performed as described above, were cut off from the base of the hypocotyl. Harvested roots were then used for immunoprecipitation and tandem mass spectrometry, and follow-up statistical analysis was done as previously reported (Wendrich et al. 2017). Enriched proteins were normalized to 35S:YFP roots following the same DEX treatment. Each transgenic line had 3 biological replicates. Arabidopsis TAIR10 database was used for data search.
Protoplast isolation and transfection for BiFC and co-localization analyses
For the bimolecular fluorescence complementation (BiFC) assays, Arabidopsis leaf mesophyll protoplasts were isolated and transfected for transient gene expression, as previously reported (Wu et al. 2009; Yoo et al. 2007). After overnight incubation in darkness following transfection with respective plasmids (Table S10), fluorescence signals from protoplasts were observed, and frequencies of observations were counted under a Zeiss Axio Imager microscope. Representative images were taken by a Zeiss LSM700 confocal laser scanning microscope.
Tobacco leaf infiltration for co-localization analyses
The respective plasmids (Table S10) were transformed into Agrobacterium tumefaciens strain GV3101 for transient expression in Nicotiana benthamiana leaves. The infiltration of tobacco leaves was done as previously described (Sparkes et al. 2006). 2 days following infiltration, gene expression via reporter fluorescence signal was confirmed under the Zeiss Axio Imager microscope, and images were taken by a Zeiss LSM700 confocal laser scanning microscope.
Immunoprecipitation assays
For both co-immunoprecipitation (Co-IP) and immunoprecipitation assays, Arabidopsis stable lines indicated in the respective figures and Table S9 were used. pRPS5A:WOX2-YFP-GR/35S:CDC48A-FLAG is the result of a cross between pRPS5A:WOX2-YFP-GR and 35S:CDC48A-FLAG. About 1 g of 6-day-old seedlings per transgenic line was ground in liquid nitrogen with a mortar and pestle. 1 ml cold lysis buffer was added to the mortar and ground thoroughly after it was thawed. The protocol provided by µMACS™ Epitope Tag Protein Isolation Kit (Miltenyi Biotec) was followed afterward. Before adding anti-GFP, anti-FLAG beads, or anti-Tubulin beads, 50 µl lysate was taken as the input. For western blots, the inputs and immunoprecipitated samples were loaded on 8–12% PAGE gels, depending on the protein size. Then, the blots were probed with anti-GFP (ab290), anti-FLAG (Sigma), or anti-tubulin (Sigma) antibodies, each with a 1:5000 dilution.
Nomarski microscopy
For Nomarski microscopy (or DIC microscopy) analyses, siliques were first dissected on a double-sided sticky tape and cut open along the replum under a stereomicroscope using a fine needle. Samples were cleared in chloral hydrate solution (80 g chloral hydrate, 30 g H2O, and 10 g glycerol dissolved for 24 h with gentle stirring at 4 °C) on a microscope slide for 2–20 h at room temperature, depending on the embryo stage to be observed. Cleared samples were then observed and imaged under a Zeiss Axioscope2 using the 40× objective.
Confocal laser scanning microscopy
Developing seeds were dissected from siliques under a stereomicroscope and then mounted on microscope slides with 10% glycerol. Seeds were gently squeezed under the coverslip to extract embryos. Fluorescence was visualized with a Zeiss LSM700 confocal laser scanning microscope under the 40× objective, and images were captured with the ZEN 2010 software program. FM4-64 counterstaining of embryos was done as previously described (Rigal et al. 2015). For observing pRPS5A:WOX2-YFP-GR in the root, root tips cut off from seedlings were mounted in 50 µM propidium iodide (PI) and left to incubate at room temperature for 2 min. The 10× objective was used to observe whole root tips, and the 40× objective was used to discern and image reporter subcellular localization. The settings for excitation/emission were 448 nm/550–570 nm (YFP/GFP) and 550 nm/ > 610 nm (tdTomato/mCherry/PI/FM4-64). For multi-channel imaging, each channel was captured simultaneously.
Quantification and statistical analyses
Pairwise comparisons for categorical data were done by Fisher’s exact tests. p-values less than 0.05 were considered statistically significant. Bonferroni correction was applied for multiple comparisons. Mean and integrated signal intensities were quantified with ImageJ version 1.53t (imagej.net) from a single focal plane for fluorescence intensities. Quantitative data were analyzed by the Student’s t test or ANOVA with the relevant Post Hoc test for multiple comparisons using GraphPad Prism version 9.4 (GraphPad Software, Inc.). All data were plotted using GraphPad Prism version 9.4. The statistical tests used for each experiment are labeled in the relevant figure captions.
Data availability
All the data in this study are included in the figures and tables.
References
Aker J, de Vries SC (2008) Plasma membrane receptor complexes. Plant Physiol 147:1560–1564
Aoyama T, Chua NH (1997) A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J 11:605–612
Bae H, Choi SM, Yang SW, Pai H-S, Kim WT (2009) Suppression of the ER-localized AAA ATPase NgCDC48 inhibits tobacco growth and development. Mol Cells 28:57–65
Baek GH, Cheng H, Choe V, Bao X, Shao J, Luo S, Rao H (2013) Cdc48: a swiss army knife of cell biology. J Amino Acids 2013:183421
Bäurle I, Laux T (2003) Apical meristems: the plant’s fountain of youth. BioEssays 25:961–970
Begue H, Jeandroz S, Blanchard C, Wendehenne D, Rosnoblet C (2017) Structure and functions of the chaperone-like p97/CDC48 in plants. Biochim Biophys Acta Gen Subj 1861:3053–3060
Beyer HM, Gonschorek P, Samodelov SL, Meier M, Weber W, Zurbriggen MD (2015) AQUA cloning: a versatile and simple enzyme-free cloning approach. PLoS ONE 10:e0137652
Bodnar NO, Rapoport TA (2017) Molecular mechanism of substrate processing by the Cdc48 ATPase complex. Cell 169:722-735.e729
Breuninger H, Rikirsch E, Hermann M, Ueda M, Laux T (2008) Differential expression of WOX genes mediates apical-basal axis formation in the Arabidopsis embryo. Dev Cell 14:867–876
Capron A, Chatfield S, Provart N, Berleth T (2009) Embryogenesis: pattern formation from a single cell. Arabidopsis Book 7:e0126
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Copeland C, Woloshen V, Huang Y, Li X (2016) AtCDC48A is involved in the turnover of an NLR immune receptor. Plant J 88:294–305
De Rybel B, van den Berg W, Lokerse A, Liao CY, van Mourik H, Moller B, Peris CL, Weijers D (2011) A versatile set of ligation-independent cloning vectors for functional studies in plants. Plant Physiol 156:1292–1299
Dolzblasz A, Nardmann J, Clerici E, Causier B, van der Graaff E, Chen J, Davies B, Werr W, Laux T (2016) Stem cell regulation by Arabidopsis WOX genes. Mol Plant 9:1028–1039
Feiler HS, Desprez T, Santoni V, Kronenberger J, Caboche M, Traas J (1995) The higher plant Arabidopsis thaliana encodes a functional CDC48 homologue which is highly expressed in dividing and expanding cells. EMBO J 14:5626–5637
Gallois JL, Nora FR, Mizukami Y, Sablowski R (2004) WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem. Genes Dev 18:375–380
Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA 3rd, Smith HO (2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345
Haecker A, Groß-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, Laux T (2004) Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 131:657–668
Han B, Yang Z, Samma MK, Wang R, Shen W (2013) Systematic validation of candidate reference genes for qRT-PCR normalization under iron deficiency in Arabidopsis. Biometals 26:403–413
Hofmann F, Schon MA, Nodine MD (2019) The embryonic transcriptome of Arabidopsis thaliana. Plant Reprod 32:77–91
Koncz C, Schell J (1986) The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204:383–396
Laux T, Wurschum T, Breuninger H (2004) Genetic regulation of embryonic pattern formation. Plant Cell 16(Suppl):S190-202
Lenhard M, Laux T (2003) Stem cell homeostasis in the Arabidopsis shoot meristem is regulated by intercellular movement of CLAVATA3 and its sequestration by CLAVATA1. Development 130:3163–3173
Lu L, Holt A, Chen X, Liu Y, Knauer S, Tucker EJ, Sarkar AK, Hao Z, Roodbarkelari F, Shi J, Chen J, Laux T (2023) miR394 enhances WUSCHEL-induced somatic embryogenesis in Arabidopsis thaliana. New Phytol 238:1059–1072
Mayer KFX, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T (1998) Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95:805–815
Merai Z, Chumak N, Garcia-Aguilar M, Hsieh TF, Nishimura T, Schoft VK, Bindics J, Slusarz L, Arnoux S, Opravil S, Mechtler K, Zilberman D, Fischer RL, Tamaru H (2014) The AAA-ATPase molecular chaperone Cdc48/p97 disassembles sumoylated centromeres, decondenses heterochromatin, and activates ribosomal RNA genes. Proc Natl Acad Sci U S A 111:16166–16171
Meyer H, Bug M, Bremer S (2012) Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat Cell Biol 14:117–123
Ndoja A, Cohen RE, Yao T (2014) Ubiquitin signals proteolysis-independent stripping of transcription factors. Mol Cell 53:893–903
Negin B, Shemer O, Sorek Y, Eshed Williams L (2017) Shoot stem cell specification in roots by the WUSCHEL transcription factor. PLoS ONE 12:e0176093
Niehl A, Amari K, Gereige D, Brandner K, Mely Y, Heinlein M (2012) Control of Tobacco mosaic virus movement protein fate by CELL-DIVISION-CYCLE protein48. Plant Physiol 160:2093–2108
Park S, Rancour DM, Bednarek SY (2007) Protein domain-domain interactions and requirements for the negative regulation of Arabidopsis CDC48/p97 by the plant ubiquitin regulatory X (UBX) domain-containing protein, PUX1. J Biol Chem 282:5217–5224
Park S, Rancour DM, Bednarek SY (2008) In planta analysis of the cell cycle-dependent localization of AtCDC48A and its critical roles in cell division, expansion, and differentiation. Plant Physiol 148:246–258
Ramadan K, Halder S, Wiseman K, Vaz B (2017) Strategic role of the ubiquitin-dependent segregase p97 (VCP or Cdc48) in DNA replication. Chromosoma 126:17–32
Rancour DM, Dickey CE, Park S, Bednarek SY (2002) Characterization of AtCDC48. Evidence for multiple membrane fusion mechanisms at the plane of cell division in plants. Plant Physiol 130:1241–1253
Rieu I, Powers SJ (2009) Real-time quantitative RT-PCR: design, calculations, and statistics. Plant Cell 21:1031–1033
Rigal A, Doyle SM, Robert S (2015) Live cell imaging of FM4-64, a tool for tracing the endocytic pathways in Arabidopsis root cells. Methods Mol Biol 1242:93–103
Schoof H, Lenhard M, Haecker A, Mayer KFX, Jürgens G, Laux T (2000) The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100:635–644
Sparkes IA, Runions J, Kearns A, Hawes C (2006) Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat Protoc 1:2019–2025
Tucker MR, Hinze A, Tucker EJ, Takada S, Jurgens G, Laux T (2008) Vascular signalling mediated by ZWILLE potentiates WUSCHEL function during shoot meristem stem cell development in the Arabidopsis embryo. Development 135:2839–2843
van der Graaff E, Laux T, Rensing SA (2009) The WUS homeobox-containing (WOX) protein family. Genome Biol 10:248
Walter M, Chaban C, Schutze K, Batistic O, Weckermann K, Nake C, Blazevic D, Grefen C, Schumacher K, Oecking C, Harter K, Kudla J (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40:428–438
Weijers D, Franke-van Dijk M, Vencken RJ, Quint A, Hooykaas P, Offringa R (2001) An Arabidopsis minute-like phenotype caused by a semi-dominant mutation in a RIBOSOMAL PROTEIN S5 gene. Development 128:4289–4299
Wendrich JR, Boeren S, Moller BK, Weijers D, De Rybel B (2017) In vivo identification of plant protein complexes using IP–MS/MS. Methods Mol Biol 1497:147–158
Wilcox AJ, Laney JD (2009) A ubiquitin-selective AAA-ATPase mediates transcriptional switching by remodelling a repressor–promoter DNA complex. Nat Cell Biol 11:1481–1486
Wu FH, Shen SC, Lee LY, Lee SH, Chan MT, Lin CS (2009) Tape-Arabidopsis sandwich – a simpler Arabidopsis protoplast isolation method. Plant Methods 5:16
Yadav RK, Perales M, Gruel J, Girke T, Jonsson H, Reddy GV (2011) WUSCHEL protein movement mediates stem cell homeostasis in the Arabidopsis shoot apex. Genes Dev 25:2025–2030
Yang L, Zhu M, Yang Y, Wang K, Che Y, Yang S, Wang J, Yu X, Li L, Wu S, Palme K, Li X (2022) CDC48B facilitates the intercellular trafficking of SHORT-ROOT during radial patterning in roots. J Integr Plant Biol 64:843–858
Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2:1565–1572
Yoshida S, Barbier de Reuille P, Lane B, Bassel GW, Prusinkiewicz P, Smith RS, Weijers D (2014) Genetic control of plant development by overriding a geometric division rule. Dev Cell 29:75–87
Zhang Z, Tucker E, Hermann M, Laux T (2017) A molecular framework for the embryonic initiation of shoot meristem stem cells. Dev Cell 40(264–277):e264
Zuo J, Niu QW, Frugis G, Chua NH (2002) The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J 30:349–359
Acknowledgements
We are grateful to Dr. Sookhee Park for sharing the cdc48a-1/+, cdc48a-2/+, cdc48a-3/+, and pCDC48A:YFP-CDC48A cdc48a-1 seeds, Dr. Thomas Kretsch for the 35S:nYFP-ASK1 and 35S:cYFP-EID1 plasmids, Dr. Zhongjuan Zhang for the pRPS5A:WOX2-YFP-GR line, and Prof. Dr. Thomas Ott for the 35S:ER-mCherry plasmid. We also thank the members of the Laux lab for their helpful comments on the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by the German Research Foundation (DFG) under Germany’s Excellence Strategy (CIBSS–EXC-2189–Project ID390939984) and by grants (La606/17-2/19-1/20-1) to T.L.
Author information
Authors and Affiliations
Contributions
W.G. and D.T.B. designed and performed the experiments. J.R.W. and D.W. carried out the IP–MS/MS. T.L. supervised the project. D.T.B., W.G., and T.L. wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Communicated by Zhanyuan Jon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gong, W., Bak, D.T., Wendrich, J.R. et al. CDC48A, an interactor of WOX2, is required for embryonic patterning in Arabidopsis thaliana. Plant Cell Rep 43, 174 (2024). https://doi.org/10.1007/s00299-024-03158-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00299-024-03158-2