Abstract
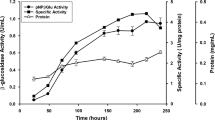
β-Glucuronidase from Penicillium purpurogenum Li-3 (PGUS) can efficiently hydrolyze glycyrrhizin into the more valuable glycyrrhetic acid monoglucuronide. However, a low productivity of PGUS and the lack of an effective separation strategy have significantly limited its industrial applications. Therefore, the production of PGUS has been improved by optimizing both the fermentation and purification strategies. A two-stage fermentation strategy was developed where PGUS was first grown with glucose and then PGUS was produced in the presence of glycyrrhizin as an inducer. By using this strategy, the biomass was increased 1.5 times and the PGUS activity increased 5.4 times compared to that when glycyrrhizin was used as the sole carbon source. The amount of PGUS produced was increased another 16.6% when the fermentation was expanded to a 15-L fermenter. An effective protocol was also established to purify the PGUS using a sequential combination of hydrophobic, strong anionexchange and gel filtration chromatography. This protocol had a recovery yield of 6% and gave PGUS that was 39 times purer than the crude PGUS. The purified PGUS had a specific activity of 350 U·mg–1.

Similar content being viewed by others
References
Makino T, Okajima K, Uebayashi R, Ohtake N, Inoue K, Mizukami H. 3-Monoglucuronyl-glycyrrhretinic acid is a substrate of organic anion transporters expressed in tubular epithelial cells and plays important roles in licorice-induced pseudoaldosteronism by inhibiting 11 β-hydroxysteroid dehydrogenase 2. Journal of Pharmacology and Experimental Therapeutics, 2012, 342(2): 297–304
Seki H, Ohyama K, Sawai S, Mizutani M, Ohnishi T, Sudo H, Akashi T, Aoki T, Saito K, Muranaka T. Licorice β-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(37): 14204–14209
Akao T. Differences in the metabolism of glycyrrhizin, glycyrrhetic acid and glycyrrhetic acid monoglucuronide by human intestinal flora. Biological & Pharmaceutical Bulletin, 2000, 23(12): 1418–1423
Matsui S, Matsumoto H, Sonoda Y, Ando K, Aizu-Yokota E, Sato T, Kasahara T. Glycyrrhizin and related compounds down-regulate production of inflammatory chemokines IL-8 and eotaxin 1 in a human lung fibroblast cell line. International Immunopharmacology, 2004, 4(13): 1633–1644
Doll R, Hill I D, Hutton C, Underwood D J V II. Clinical trial of a triterpenoid liquorice compound in gastric and duodenal ulcer. Lancet, 1962, 280(7260): 793–796
Pompei R, Flore O, Marccialis M A, Pani A, Loddo B. Glycyrrhizic acid inhibits virus growth and inactivates virus-particles. Nature, 1979, 281(5733): 689–690
Shiota G, Harada K, Ishida M, Tomie Y, Okubo M, Katayama S, Ito H, Kawasaki H. Inhibition of hepatocellular carcinoma by glycyrrhizin in diethylnitrosamine-treated mice. Carcinogenesis, 1999, 20(1): 59–63
Isbrucker R A, Burdock G A. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regulatory Toxicology and Pharmacology, 2006, 46(3): 167–192
Feng S J, Li C, Xu X L, Wang X Y. Screening strains for directed biosynthesis of β-D-mono-glucuronide-glycyrrhizin and kinetics of enzyme production. Journal of Molecular Catalysis. B, Enzymatic, 2006, 43(1–4): 63–67
Zou S P, Zhou J J, Kaleem I, Xie L P, Liu G Y, Li C. Preparative enrichment and separation of glycyrrhetinic acid monoglucuronide from fermentation broths with macroporous resins. Separation Science and Technology, 2012, 47(7): 1055–1062
Zou S P, Liu G Y, Kaleem I, Li C. Purification and characterization of a highly selective glycyrrhizin-hydrolyzing β-glucuronidase from Penicillium purpurogenum Li-3. Process Biochemistry, 2013, 48(2): 358–363
Qi F, Kaleem I, Lv B, Guo X, Li C. Enhancement of recombinant β-D-glucuronidase production under low-shear modeled microgravity in Pichia pastoris. Journal of Chemical Technology and Biotechnology, 2011, 86(4): 505–511
Mizutani K, Kuramoto T, Tamura Y, Ohtake N, Doi S, Nakaura M, Tanaka O. Sweetness of glycyrrhetic acid 3-O-β-D-monoglucuronide and the related glycosides. Bioscience, Biotechnology, and Biochemistry, 1994, 58(3): 554–555
Park H Y, Park S H, Yoon H K, Han M J, Kim D H. Anti-allergic activity of 18 β-glycyrrhetinic acid-3-O-β-D-glucuronide. Archives of Pharmacal Research, 2004, 27(1): 57–60
Maitraie D, Hung C F, Tu H Y, Liou Y T, Wei B L, Yang S C, Wang J P, Lin C N. Synthesis, anti-inflammatory, and antioxidant activities of 18 β-glycyrrhetinic acid derivatives as chemical mediators and xanthine oxidase inhibitors. Bioorganic & Medicinal Chemistry, 2009, 17(7): 2785–2792
Zou S, Guo S, Kaleem I, Li C. Purification, characterization and comparison of Penicillium purpurogenum β-glucuronidases expressed in Escherichia coli and Pichia pastoris. Journal of Chemical Technology and Biotechnology, 2013, 88(10): 1913–1919
Song X, Jiang Z, Li L, Wu H. Immobilization of β-glucuronidase in lysozyme-induced biosilica particles to improve its stability. Frontiers of Chemical Science and Engineering, 2014, 8(3): 353–361
Kim D H, Jin Y H, Jung E A, Han M J, Kobashi K. Purification and characterization of β-glucuronidase from Escherichia coli HGU-3, a human intestinal bacterium. Biological & Pharmaceutical Bulletin, 1995, 18(9): 1184–1188
Akao T. Competition in the metabolism of glycyrrhizin with glycyrrhetic acid mono-glucuronide by mixed Eubacterium sp GLH and Ruminococcus sp PO1-3. Biological & Pharmaceutical Bulletin, 2000, 23(2): 149–154
Amin H A S, El-Menoufy H A, El-Mehalawy A A, Mostafa E S. Biosynthesis of glycyrrhetinic acid 3-O-mono-β-D-glucuronide by free and immobilized Aspergillus terreus β-D-glucuronidase. Journal of Molecular Catalysis. B, Enzymatic, 2011, 69(1–2): 54–59
Kuramoto T, Ito Y, Oda M, Tamura Y, Kitahata S. Microbialproduction of glycyrrhetic acid 3-O-mono-β-D-glucuronide from glycyrrhizin by Cryptococcus-magnus MG-27. Bioscience, Biotechnology, and Biochemistry, 1994, 58(3): 455–458
Lu D Q, Li H, Dai Y, Ouyang P K. Biocatalytic properties of a novel crude glycyrrhizin hydrolase from the liver of the domestic duck. Journal of Molecular Catalysis. B, Enzymatic, 2006, 43(1–4): 148–152
Bradford M M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Analytical Biochemistry, 1976, 72(1–2): 248–254
Papagianni M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnology Advances, 2004, 22(3): 189–259
Posch A E, Herwig C, Spadiut O. Science-based bioprocess design for filamentous fungi. Trends in Biotechnology, 2013, 31(1): 37–44
Choonia H S, Saptarshi S D, Lele S S. Release of intracellular β-galactosidase from Lactobacillus acidophilus and L-asparaginase from Pectobacterium carotovorum by high-pressure homogenization. Chemical Engineering Communications, 2013, 200(11): 1415–1424
Liu Y, Pietzsch M, Ulrich J. Purification of L-asparaginase II by crystallization. Frontiers of Chemical Science and Engineering, 2013, 7(1): 37–42
Park H Y, Kim N Y, Han M J, Bae E A, Kim D H. Purification and characterization of two novel β-D-glucuronidases converting glycyrrhizin to 18 β-glycyrrhetinic acid-3-O-β-D-glucuronide from Streptococcus LJ-22. Journal of Microbiology and Biotechnology, 2005, 15(4): 792–799
Kuroyama H, Tsutsui N, Hashimoto Y, Tsumuraya Y. Purification and characterization of a β-glucuronidase from Aspergillus niger. Carbohydrate Research, 2001, 333(1): 27–39
Sakurama H, Kishino S, Uchibori Y, Yonejima Y, Ashida H, Kita K, Takahashi S, Ogawa J. β-Glucuronidase from Lactobacillus brevis useful for baicalin hydrolysis belongs to glycoside hydrolase family 30. Applied Microbiology and Biotechnology, 2014, 98(9): 4021–4032
Nguyen Q D, Rezessy-Szabo J M, Bhat M K, Hoschke A. Purification and some properties of β-fructofuranosidase from Aspergillus niger IMI303386. Process Biochemistry, 2005, 40(7): 2461–2466
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, S., Feng, X. & Li, C. Enhanced production of β-glucuronidase from Penicillium purpurogenum Li-3 by optimizing fermentation and downstream processes. Front. Chem. Sci. Eng. 9, 501–510 (2015). https://doi.org/10.1007/s11705-015-1544-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-015-1544-0