Abstract
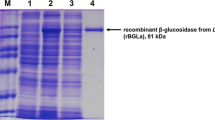
Baicalin (baicalein 7-O-β-d-glucuronide) is one of the major flavonoid glucuronides found in traditional herbal medicines. Because its aglycone, baicalein, is absorbed more quickly and shows more effective properties than baicalin, the conversion of baicalin into baicalein by β-glucuronidase (GUS) has drawn the attention of researchers. Recently, we have found that Lactobacillus brevis subsp. coagulans can convert baicalin to baicalein. Therefore, we aimed to identify and characterize the converting enzyme from L. brevis subsp. coagulans. First, we purified this enzyme from the cell-free extracts of L. brevis subsp. coagulans and cloned its gene. Surprisingly, this enzyme was found to be a GUS belonging to glycoside hydrolase (GH) family 30 (designated as LcGUS30), and its amino acid sequence has little similarity with any GUS belonging to GH families 1, 2, and 79 that have been reported so far. We then established a high-level expression and simple purification system of the recombinant LcGUS30 in Escherichia coli. The detailed analysis of the substrate specificity revealed that LcGUS30 has strict specificity toward glycon but not toward aglycones. Interestingly, LcGUS30 prefers baicalin rather than estrone 3-(β-d-glucuronide), one of the human endogenous steroid hormones. These results indicated that L. brevis subsp. coagulans and LcGUS30 should serve as powerful tools for the construction of a safe bioconversion system for baicalin. In addition, we propose that this novel type of GUS forms a new group in subfamily 3 of GH family 30.






Similar content being viewed by others
References
Adlercreutz H, Martin F, Pulkkinen M, Dencker H, Rimér U, Sjöberg NO, Tikkanen MJ (1976) Intestinal metabolism of estrogens. J Clin Endocrinol Metab 43:497–505. doi:10.1210/jcem-43-3-497
Adlercreutz H, Martin F, Järvenpää P, Fotsis T (1979) Steroid absorption and enterohepatic recycling. Contraception 20:201–223. doi:10.1016/0010-7824(79)90094-5
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi:10.1093/nar/25.17.3389
Aspeborg H, Coutinho PM, Wang Y, Brumer H 3rd, Henrissat B (2012) Evolution, substrate specificity and subfamily classification of glycoside hydrolase family 5 (GH5). BMC Evol Biol 12:186. doi:10.1186/1471-2148-12-186
Bowers LD, Johnson PR (1981) Characterization of immobilized β-glucuronidase in aqueous and mixed solvent systems. Biochim Biophys Acta 661:100–105. doi:10.1016/0005-2744(81)90087-5
Brumshtein B, Greenblatt HM, Butters TD, Shaaltiel Y, Aviezer D, Silman I, Futerman AH, Sussman JL (2007) Crystal structures of complexes of N-butyl- and N-nonyl-deoxynojirimycin bound to acid β-glucosidase: insights into the mechanism of chemical chaperone action in Gaucher disease. J Biol Chem 282:29052–29058. doi:10.1074/jbc.M705005200
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37:D233–D238. doi:10.1093/nar/gkn663
Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro-O M, Huang CL (2008) Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci U S A 105:9805–9810. doi:10.1073/pnas.0803223105
Finn RD, Mistry J, Tate J, Coggill P, Heger A, Pollington JE, Gavin OL, Gunesekaran P, Ceric G, Forslund K, Holm L, Sonnhammer EL, Eddy SR, Bateman A (2010) The Pfam protein families database. Nucleic Acids Res 38:D211–D222. doi:10.1093/nar/gkr1065
Graef V, Furuya E, Nishikaze O (1977) Hydrolysis of steroid glucuronides with beta-glucuronidase preparations from bovine liver, Helix pomatia, and E. coli. Clin Chem 23:532–535
Ikemoto S, Sugimura K, Yoshida N, Yasumoto R, Wada S, Yamamoto K, Kishimoto T (2000) Antitumor effects of Scutellariae radix and its components baicalein, baicalin, and wogonin on bladder cancer cell lines. Urology 55:951–955. doi:10.1016/S0090-4295(00)00467-2
Inoue H, Nojima H, Okayama H (1990) High efficiently transformation of Escherichia coli with plasmids. Gene 96:23–28. doi:10.1016/0378-1119(90)90336-P
Kim HS, Kim JY, Park MS, Zheng H, Ji GE (2009) Cloning and expression of β-glucuronidase from Lactobacillus brevis in E. coli and application in bioconversion of baicalin and wogonoside. J Microb Biotechnol 19:1650–1655
Kishi A, Uno K, Matsubara Y, Okuda C, Kishida T (1996) Effect of the oral administration of Lactobacillus brevis subsp. coagulans on interferon-alpha producing capacity in humans. J Am Coll Nutr 15:408–412
Kobashi K, Akao T (1997) Relation of intestinal bacteria to pharmacological effect of glycosides. Biosci Microflora 16:1–7
Kubo M, Kimura Y, Odani T, Tani T, Namba K (1981) Studies on Scutellariae radix. Part II: the antibacterial substance. Planta Med 43:194–201
Kuhn JG (1998) Pharmacology of irinotecan. Oncology 12:39–42
Lai MY, Hsiu SL, Tsai SY, Hou YC, Chao PD (2003) Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J Pharm Pharmacol 55:205–209. doi:10.1211/002235702522
Liu JJ, Huang TS, Cheng WF, Lu FJ (2003) Baicalein and baicalin are potent inhibitors of angiogenesis: inhibition of endothelial cell proliferation, migration and differentiation. Int J Cancer 106:559–565. doi:10.1002/ijc.11267
Middleton E Jr, Kandaswami C, Theoharides TC (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 52:673–751
Rémy-Martin A, Prost O, Nicollier M, Burnod J, Adessi GL (1983) Estrone sulfate concentrations in plasma of normal individuals, postmenopausal women with breast cancer, and men with cirrhosis. Clin Chem 29:86–89
Roy AB (1970) Enzymological aspects of steroid conjugation. In: Bernstein S, Soloman S (eds) Chemical and biological aspects of steroid conjunction. Springer, New York, pp 74–130
Russell WM, Klaenhammer TR (2001) Identification and cloning of gusA, encoding a new β-glucuronidase from Lactobacillus gasseri ADH. Appl Environ Microbiol 67:1253–1261. doi:10.1128/AEM.67.3.1253-1261.2001
Sasaki K, Taura F, Shoyama Y, Morimoto S (2000) Molecular characterization of a novel β-glucuronidase from Scutellaria baicalensis Georgi. J Biol Chem 275:27466–27472. doi:10.1074/jbc.M004674200
Shen YC, Chiou WF, Chou YC, Chen CF (2003) Mechanisms in mediating the anti-inflammatory effects of baicalin and baicalein in human leukocytes. Eur J Pharmacol 465:171–181. doi:10.1016/S0014-2999(03)01378-5
Simons AL, Renouf M, Hendrich S, Murphy PA (2005) Human gut microbial degradation of flavonoids: structure-function relationships. J Agric Food Chem 53:4258–4263. doi:10.1021/jf0500177
St John FJ, González JM, Pozharski E (2010) Consolidation of glycosyl hydrolase family 30: a dual domain 4/7 hydrolase family consisting of two structurally distinct groups. FEBS Lett 584:4435–4441. doi:10.1016/j.febslet.2010.09.051
Tohyama O, Imura A, Iwano A, Freund JN, Henrissat B, Fujimori T, Nabeshima Y (2004) Klotho is a novel β-glucuronidase capable of hydrolyzing steroid β-glucuronides. J Biol Chem 279:9777–9784. doi:10.1074/jbc.M312392200
Wallace BD, Wang H, Lane KT, Scott JE, Orans J, Koo JS, Venkatesh M, Jobin C, Yeh LA, Mani S, Redinbo MR (2010) Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 330:831–835. doi:10.1126/science.1191175
Walle T (2004) Absorption and metabolism of flavonoids. Free Radic Biol Med 36:829–837. doi:10.1016/j.freeradbiomed.2004.01.002
Wilson KJ, Hughes SG, Jefferson RA (1992) The Escherichia coli gus operon: induction and expression of the gus operon in E. coli and the occurrence and use of GUS in other bacteria. In: Gallagher SR (ed) GUS protocols. Using the GUS gene as reporter of gene expression. Academic, San Diego, pp 7–22
Xing J, Chen X, Zhong D (2005) Absorption and enterohepatic circulation of baicalin in rats. Life Sci 78:140–146. doi:10.1016/j.lfs.2005.04.072
Yim JS, Kim YS, Moon SK, Cho KH, Bae HS, Kim JJ, Park EK, Kim DH (2004) Metabolic activities of ginsenoside Rb1, baicalin, glycyrrhizin and geniposide to their bioactive compounds by human intestinal microflora. Biol Pharm Bull 27:1580–1593. doi:10.1248/bpb.27.1580
Zhang C, Zhang Y, Chen J, Liang X (2005) Purification and characterization of baicalin-β-d-glucuronidase hydrolyzing baicalin to baicalein from fresh roots of Scutellaria viscidula Bge. Process Biochem 40:1911–1915. doi:10.1016/j.procbio.2004.07.003
Zhao Y, Li H, Gao Z, Gong Y, Xu H (2006) Effects of flavonoids extracted from Scutellaria baicalensis Georgi on hemin-nitrite-H2O2 induced liver injury. Eur J Pharmacol 536:192–199. doi:10.1016/j.ejphar.2006.02.045
Author information
Authors and Affiliations
Corresponding author
Additional information
Haruko Sakurama and Shigenobu Kishino contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sakurama, H., Kishino, S., Uchibori, Y. et al. β-Glucuronidase from Lactobacillus brevis useful for baicalin hydrolysis belongs to glycoside hydrolase family 30. Appl Microbiol Biotechnol 98, 4021–4032 (2014). https://doi.org/10.1007/s00253-013-5325-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5325-8