Abstract
Background
Serum bile acids (BAs) are elevated following bariatric surgery and have emerged as a potential glucose-lowering beneficial factor. The change of BA components and its underlying mechanisms may be of great significance during bariatric surgery. The aim of this study is to investigate the effects of different bariatric procedures on serum BA composition and explore the potential mechanisms using a diabetic rat model.
Methods
Duodenal-jejunal bypass (DJB), sleeve gastrectomy (SG), and sham operation were performed in diabetic rats induced by high-fat diet (HFD) and streptozotocin (STZ). Body weight, food intake, oral glucose tolerance test (OGTT), and insulin tolerance test (ITT) were measured at indicated time points. Serum BAs composition and the expression of cholesterol 7α hydroxylase (CYP7A1), bile acid: CoA synthase (BACS) and bile acid-CoA: amino acid N-acyltransferase (BAAT) at both transcriptional and protein levels in the liver were evaluated at 12 weeks postoperatively.
Results
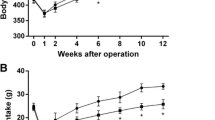
Compared with sham group, DJB and SG both achieved rapid and sustained improvements in glucose tolerance and insulin sensitivity. They also resulted in increased serum BAs, especially the taurine-conjugated BAs by elevated conjugation. No obvious difference was detected between DJB and SG except that SG achieved decreased weight gain and food intake.
Conclusions
The preferentially elevated serum taurine-conjugated BAs were similar after different bariatric surgeries, and the enhanced conjugation of BAs in the liver might account for the changed serum BAs profiles.






Similar content being viewed by others
References
Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–21.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–56. e5.
Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219–34.
Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238(4):467–84. discussion 84–5.
Wang TT, Hu SY, Gao HD, et al. Ileal transposition controls diabetes as well as modified duodenal jejunal bypass with better lipid lowering in a nonobese rat model of type II diabetes by increasing GLP-1. Ann Surg. 2008;247(6):968–75.
Liu S, Zhang G, Wang L, et al. The entire small intestine mediates the changes in glucose homeostasis after intestinal surgery in Goto-Kakizaki rats. Ann Surg. 2012;256(6):1049–58.
Sun D, Liu S, Zhang G, et al. Sub-sleeve gastrectomy achieves good diabetes control without weight loss in a non-obese diabetic rat model. Surg Endosc. 2014;28(3):1010–8.
Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery worldwide 2013. Obes Surg. 2015
Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252(2):319–24.
Kohli R, Bradley D, Setchell KD, et al. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98(4):E708–12.
Cummings BP, Bettaieb A, Graham JL, et al. Bile-acid-mediated decrease in endoplasmic reticulum stress: a potential contributor to the metabolic benefits of ileal interposition surgery in UCD-T2DM rats. Dis Model Mech. 2013;6(2):443–56.
Myronovych A, Kirby M, Ryan KK, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring). 2014;22(2):390–400.
Han H, Hu C, Wang L, et al. Duodenal-jejunal bypass surgery suppresses hepatic de novo lipogenesis and alleviates liver fat accumulation in a diabetic rat model. Obes Surg. 2014;24(12):2152–60.
de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17(5):657–69.
Li T, Chiang JYL. Bile acids as metabolic regulators. Curr Opin Gastroenterol. 2015;31(2):159–65.
Fiorucci S, Mencarelli A, Palladino G, et al. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol Sci. 2009;30(11):570–80.
Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–9.
Patriti A, Facchiano E, Donini A. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;240(2):388–9. author reply 9–91.
Srinivasan K, Viswanad B, Asrat L, et al. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52(4):313–20.
Pereferrer FS, Gonzalez MH, Rovira AF, et al. Influence of sleeve gastrectomy on several experimental models of obesity: metabolic and hormonal implications. Obes Surg. 2008;18(1):97–108.
Bruinsma BG, Uygun K, Yarmush ML, et al. Surgical models of Roux-en-Y gastric bypass surgery and sleeve gastrectomy in rats and mice. Nat Protoc. 2015;10(3):495–507.
Kawasaki T, Ohta M, Kawano Y, et al. Effects of sleeve gastrectomy and gastric banding on the hypothalamic feeding center in an obese rat model. Surg Today. 2015;45(12):1560–6.
Zwicker BL, Agellon LB. Transport and biological activities of bile acids. Int J Biochem Cell Biol. 2013;45(7):1389–98.
Li T, Owsley E, Matozel M, et al. Transgenic expression of cholesterol 7 alpha-hydroxylase in the liver prevents high-fat diet induced obesity and insulin resistance in mice. Hepatology. 2010;52(2):678–90.
Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83(2):633–71.
Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009;50(12):2340–57.
Ferrannini E, Camastra S, Astiarraga B, et al. Increased bile acid synthesis and deconjugation after biliopancreatic diversion. Diabetes. 2015;64(10):3377–85.
Kohli R, Kirby M, Setchell KD, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol. 2010;299(3):G652–60.
Braghetto I, Davanzo C, Korn O, et al. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg. 2009;19(11):1515–21.
Sjovall J. Dietary glycine and taurine on bile acid conjugation in man; bile acids and steroids 75. Proc Soc Exp Biol Med. 1959;100(4):676–8.
Shonsey EM, Wheeler J, Johnson M, et al. Synthesis of bile acid coenzyme a thioesters in the amino acid conjugation of bile acids. Methods Enzymol. 2005;400:360–73.
Hayes KC, Sturman JA. Taurine in metabolism. Annu Rev Nutr. 1981;1:401–25.
Jeevanandam M, Ramias L, Schiller WR. Altered plasma free amino acid levels in obese traumatized man. Metabolism. 1991;40(4):385–90.
Tsuboyama-Kasaoka N, Shozawa C, Sano K, et al. Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity. Endocrinology. 2006;147(7):3276–84.
Rosa FT, Freitas EC, Deminice R, et al. Oxidative stress and inflammation in obesity after taurine supplementation: a double-blind, placebo-controlled study. Eur J Nutr. 2014;53(3):823–30.
Carneiro EA, Latorraca MQ, Araujo E, et al. Taurine supplementation modulates glucose homeostasis and islet fanction. J Nutr Biochem. 2009;20(7):503–11.
Cummings BP, Bettaieb A, Graham JL, et al. Vertical sleeve gastrectomy improves glucose and lipid metabolism and delays diabetes onset in UCD-T2DM rats. Endocrinology. 2012;153(8):3620–32.
Chong CPK, Mills PB, McClean P, et al. Bile acid-CoA ligase deficiency—a new inborn error of bile acid metabolism. J Inherit Metab Dis. 2012;35(3):521–30.
Sayin SI, Wahlstrom A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of Tauro-beta-muricholic acid, a naturally occurring FXR Antagonist. Cell Metab. 2013;17(2):225–35.
Rembacz KP, Woudenberg J, Hoekstra M, et al. Unconjugated bile salts shuttle through hepatocyte peroxisomes for taurine conjugation. Hepatology. 2010;52(6):2167–76.
Fisher E, Nitz I, Lindner I, et al. Candidate gene association study of type 2 diabetes in a nested case–control study of the EPIC-Potsdam cohort—role of fat assimilation. Mol Nutr Food Res. 2007;51(2):185–91.
Garbutt JT, Wilkins RM, Lack L, et al. Bacterial modification of taurocholate during enterohepatic recirculation in normal man and patients with small intestinal disease. Gastroenterology. 1970;59(4):553–66.
Alrefai W, Gill R. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res. 2007;24(10):1803–23.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no.81270888/H0713, no.81370496/H0308), the Fundamental Research Funds of Shandong University (no. 2014QLKY22), and the Taishan Scholar Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All study protocols were approved by the Animal Care and Utilization Committee of Shandong University.
Conflict of Interest
The authors declare that they have no competing interests.
Statement of Informed Consent
Does not apply.
Statement of Human and Animal Rights
All applicable institutional and national guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Wu, Q., Zhang, X., Zhong, M. et al. Effects of Bariatric Surgery on Serum Bile Acid Composition and Conjugation in a Diabetic Rat Model. OBES SURG 26, 2384–2392 (2016). https://doi.org/10.1007/s11695-016-2087-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2087-2