Abstract
Background
Bariatric surgery is an efficient procedure for remission of type 2 diabetes (T2DM) in morbid obesity. However, in Asian countries, mean body mass index (BMI) of T2DM patients is about 25 kg/m2. Various data on patients undergoing gastric bypass surgery showed that control of T2DM after surgery occurs rapidly and somewhat independent to weight loss. We hypothesized that in non-obese patients with T2DM, the glycemic control would be achieved as a consequence of gastric bypass surgery.
Methods
From September 2009, the 172 patients have had laparoscopic single anastomosis gastric bypass (LSAGB) surgery. Among them, 107 patients have been followed up more than 1 year. We analyzed the dataset of these patients. Values related to diabetes were measured before and 1, 2, and 3 years after the surgery.
Results
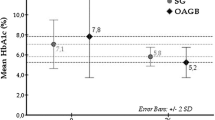
The mean BMI decreased during the first year after the surgery but plateaued after that. The mean glycosylated hemoglobin level decreased continuously. The mean fasting and postglucose loading plasma glucose level also decreased.
Conclusion
After LSAGB surgery in non-obese T2DM patients, the control of T2DM was possible safely and effectively. However, longer follow-up with matched control group is essential.

Similar content being viewed by others
References
Zimmer P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–87.
Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88.
Korea Health Statistics 2010: Korea National Health and Nutrition Examination Study, the Ministry of Health and Welfare, 2010 (http://knhanes.cdc.go.kr) (KNHANES V-1).
Chan JM, Rimm EB, Colditz GA, et al. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–9.
Colditz GA, Willett WC, Rotnitzky A, et al. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–6.
Ambady R, Ronald Ching WM, Chamukuttau S. Diabetes in Asia. Lancet. 2010;375:408–18.
Juliana CN, Vasanti M, Weiping J, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–37.
Oh JY, Hong YS, Sung YA, et al. Prevalence and factor analysis of metabolic syndrome in an urban Korean population. Diabetes Care. 2004;27:2027–32.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37.
Mingrone G, DeGaetano A, Greco AV, et al. Reversibility of insulin resistance in obese diabetic patients: role of plasma lipids. Diabetologia. 1997;40:599–605.
Dixon JB, Zimmet P, Alberti KG, et al. Bariatric surgery: an IDF statement for obese type 2 diabetes. Diabet Med. 2011;28:628–42.
Rutledge R. The mini-gastric bypass: experience with the first 1274 cases. Obes Surg. 2001;11:276–80.
Pories WJ, Albrecht RJ. Etiology of type 2 diabetes mellitus: role of the foregut. World J Surg. 2001;25:527–31.
Rubino F, Gagner M. Potential of surgery for curing type 2 diabetes mellitus. Ann Surg. 2002;236:554–9.
Scott EG, Frank LG, Stanley K. Effects of obesity surgery on non-insulin-dependent diabetes mellitus. Arch Surg. 2002;137:1109–17.
American Diabetes Association. Diabetes management in correctional institutions. Diabetes Care. 2010;33:S75–81.
Scopinaro N, Marinari G, Camerini GB, et al. Specific effects of biliopancreatic diversion on the major components of metabolic syndrome: a long-term follow-up study. Diabetes Care. 2005;28:2406–11.
Tejirian T, Jensen C, Dutson E. Bariatric surgery and type 2 diabetes mellitus: surgically induced remission. J Diabetes Sci Technol. 2008;2:685–91.
Bose M, Olivan B, Teixeira J, et al. Do incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: what are the evidence? Obes Surg. 2009;19:217–29.
Padwal RS, Gabr RQ, Sharma AM, et al. Effect of gastric bypass surgery on the absorption and bioavailability of metformin. Diabetes Care. 2011;34:1295–300.
Patti ME, Houten SM, Bianco A, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity. 2009;17:1671–7.
Laferrere B, McGinty J, Heshka S, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–16.
Rubino F, R’bibo SL, del Genio F, et al. Metabolic surgery: the role of the gastrointestinal tract in diabetes mellitus. Nat Rev Endocrinol. 2010;6:102–9.
Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–9.
Garcia-Caballero M, Valle M, Martinez-Moreno JM, et al. Resolution of diabetes mellitus and metabolic syndrome in normal weight 24-29 BMI patients with one anastomosis gastric bypass. Nutr Hosp. 2012;27:623–31.
Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–42.
Roger HU, Anna ME. Entero-insular axis. Arch Intern Med. 1969;123:261–6.
Creutzfeldt W. The incretin concept today. Diabetologia. 1979;16:75–85.
Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects. J Clin Invest. 1967;46:1954–62.
Preitner F, Ibberson M, Franglin I, et al. Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J Clin Invest. 2004;113:635–45.
Timothy JK, Joel FH. The glucagon-like peptides. Endocr Rev. 1999;20:876–913.
Fiona MG, Leanne W, Anna KS, et al. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes. 2003;52:1147–54.
Juris JM, Michael AN, Wolfgang ES, et al. Gastric inhibitory polypeptide: the neglected incretin revisited. Regul Pept. 2002;107:1–13.
Creutzfeldt W. The entero-insular axis in type 2 diabetes—incretins as therapeutic agents. Exp Clin Endocrinol Diabetes. 2001;109(Suppl2):S288–303.
Santoro S, Castro LC, Velhote MC, et al. Sleeve gastrectomy with transit bipartition. A potent intervention for metabolic syndrome and obesity. Ann Surg. 2012;256:104–10.
Vilsboll T, Krarup T, Sonne J, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:2706–13.
Vilsboll T, Krarup T, Deacon CF, et al. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–13.
Fetner R, McGinty J, Russell C, et al. Incretins, diabetes, and bariatric surgery: a review. Surg Obes Relat Dis. 2005;1:589–98.
Wang W, Wei PL, Lee YC, et al. Short-term results of laparoscopic mini-gastric bypass. Obes Surg. 2005;15(5):648–54.
Rutledge R, Walsh TR. Continued excellent results with the mini-gastric bypass: six-year study in 2,410 patients. Obes Surg. 2005;15(9):1304–8.
Piazza L, Ferrara F, Leanza S, et al. A laparoscopic mini-gastric bypass: short-term single-institute experience. Updat Surg. 2011;63(4):239–42.
Noun R, Skaff J, Riachi E, et al. One thousand consecutive mini-gastric bypass: short- and long-term outcome. Obes Surg. 2012;22(5):697–703.
Lee WJ, Wang W, Lee YC, et al. Laparoscopic mini-gastric bypass: experience with tailored bypass limb according to body weight. Obes Surg. 2008;18(3):294–9.
Dang H, Arias E, Szomstein S, et al. Laparoscopic conversion of distal mini-gastric bypass to proximal Roux-en-Y gastric bypass for malnutrition: case report and review of the literature. SORD. 2009;5:383–6.
Johnson WH, Fernanadez AZ, Farrell TM, et al. Surgical revision of loop gastric bypass procedure: multicenter review of complications and conversions to Roux-en-Y gastric bypass. SORD. 2007;3:37–41.
Azagury DE, Abu Dayyeh BK, Greenwalt IT, et al. Marginal ulceration after Roux-en-Y gastric bypass surgery: characteristics, risk factors, treatment, and outcomes. Endoscopy. 2011;43:950–4.
Kim YG, Hahn S, Oh TJ, et al. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56:696–708.
Acknowledgments
The point of this work was presented at the International Federation for the Surgery of Obesity and Metabolic disorders–Asia Pacific Chapter (IFSO-APC) meeting 2013 in Taiwan. This work was supported in part by the Soonchunhyang University Research Fund. Authors appreciate sincerely Su Yoon Go (Suzanne Burrows) for her help to revise this manuscript.
Conflict of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, M.J., Hur, K.Y. Short-Term Outcomes of Laparoscopic Single Anastomosis Gastric Bypass (LSAGB) for the Treatment of Type 2 Diabetes in Lower BMI (<30 kg/m2) Patients. OBES SURG 24, 1044–1051 (2014). https://doi.org/10.1007/s11695-014-1202-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-014-1202-5