Abstract
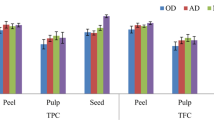
Coffee is one of the most widely used beverages in the world. Postharvest techniques mainly affect the nutritional value and bioactive components of the food samples. In this study, the effect of drying methods and extraction solvents was assessed on different parts of coffee fruit. The total polyphenol content, total flavonoid content, and the antioxidant activity in terms of DPPH scavenging and reducing power of the samples were measured. Results revealed that oven drying of the coffee fruit parts led to significantly higher total polyphenol content, total flavonoid content, and antioxidant activities compared to the shade drying at room temperature. Ethyl acetate extracts of all samples exhibited significantly lower total polyphenol content, total flavonoid content, and antioxidant activities than the methanolic extracts. Coffee beans were more potent in terms of phenolic content and antioxidant activity as compared to the coffee pulp and parchment. These results suggest that oven drying method can be adopted for processing of coffee fruit.







Similar content being viewed by others
References
USDA, Coffee: world market and trade. (2018). http://www.fas.usda.gov/data/coffee-world-markets-and-trade
F. Anthony, M.C. Combes, C. Astorga, B. Bertrand, G. Graziosi, P. Lashermes, The origin of cultivated Coffea arabica L. varieties revealed by AFLP and SSR markers. Theor. Appl. Genet. 104(5), 894–900 (2002). https://doi.org/10.1007/s00122-001-0798-8
H.A.J. Pohlan, M.J. Janssens, Growth and production of coffee, in Soils, Plant Growth and Crop Production, ed. by W.H. Verheye (EOLSS Publications, Abu Dhabi, 2010), pp. 102–134
FAOSTAT (2015) Coffee 2015. http://www.fao.org/3/a-i4985e.pdf
P. Ghosh, N. Venkatachalapathy, Processing and drying of coffee—a review. Int. J. Eng. Res. Technol. 3(12), 784–794 (2014)
H.D. Belitz, W. Grosch, P. Schieberle, Coffee, tea, cocoa, in Food Chemistry, ed. by H.D. Belitz, W. Grosch, P. Schieberle (Springer, Berlin, 2009)
D. Brand, A. Pandey, S. Roussos, C.R. Soccol, Biological detoxification of coffee husk by filamentous fungi using a solid state fermentation system. Enzyme Microb. Technol. 27(1–2), 127–133 (2000). https://doi.org/10.1016/S0141-0229(00)00186-1
A.S. Franca, Leandro S. Oliveira, Coffee processing solid wastes: current uses and future perspectives. Agric. Wastes 9, 155–189 (2009)
P.N. Navya, S.M. Pushpa, Production, statistical optimization and application of endoglucanase from Rhizopus stolonifer utilizing coffee husk. Bioprocess Biosyst. Eng. 36(8), 1115–1123 (2013). https://doi.org/10.1007/s00449-012-0865-3
A. Napolitano, V. Fogliano, A. Tafuri, A. Ritieni, Natural occurrence of ochratoxin A and antioxidant activities of green and roasted coffees and corresponding byproducts. J. Agric. Food Chem. 55(25), 10499–10504 (2007). https://doi.org/10.1021/jf071959+
A. Pourfarzad, H. Mahdavian-Mehr, N. Sedaghat, Coffee silverskin as a source of dietary fiber in bread-making: optimization of chemical treatment using response surface methodology. LWT Food Sci. Technol. 50(2), 599–606 (2013). https://doi.org/10.1016/j.lwt.2012.08.001
W. Mullen, B. Nemzer, B. Ou, A. Stalmach, J. Hunter, M.N. Clifford, E. Combet, The antioxidant and chlorogenic acid profiles of whole coffee fruits are influenced by the extraction procedures. J. Agric. Food Chem. 59(8), 3754–3762 (2011). https://doi.org/10.1021/jf200122m
K.L. Johnston, M.N. Clifford, L.M. Morgan, Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr. 78(4), 728–733 (2003)
E. Thom, The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J. Int. Med. Res. 35(6), 900–908 (2007). https://doi.org/10.1177/147323000703500620
M. Tyszka-Czochara, K. Pawel, M. Marcin, Caffeic acid expands anti-tumor effect of metformin in human metastatic cervical carcinoma HTB-34 cells: implications of AMPK activation and impairment of fatty acids de novo biosynthesis. Int. J. Mol. Sci. 18(2), 462 (2017). https://doi.org/10.3390/ijms18020462
K.S. Andrade, R.T. Gonalvez, M. Maraschin, R.M. Ribeiro-Do-Valle, J. Martínez, S.R.S. Ferreira, Supercritical fluid extraction from spent coffee grounds and coffee husks: antioxidant activity and effect of operational variables on extract composition. Talanta 88, 544–552 (2012). https://doi.org/10.1016/j.talanta.2011.11.031
L.F. Ballesteros, J.A. Teixeira, S.I. Mussatto, Selection of the solvent and extraction conditions for maximum recovery of antioxidant phenolic compounds from coffee silverskin. Food Bioprocess Technol. 7, 1322–1332 (2014). https://doi.org/10.1007/s11947-013-1115-7
G. Budryn, E. Nebesny, A. Podsȩdek, D. Zyzelewicz, M. Materska, S. Jankowski, B. Janda, Effect of different extraction methods on the recovery of chlorogenic acids, caffeine and maillard reaction products in coffee beans. Eur. Food Res. Technol. 228(6), 913–922 (2009). https://doi.org/10.1007/s00217-008-1004-x
M. Pinelo, A.G. Tress, M. Pedersen, A. Arnous, A.S. Meyer, Effect of cellulases, solvent type and particle size distribution on the extraction of chlorogenic acid and other phenols from spent coffee grounds. Am. J. Food Technol. 2(7), 641–651 (2007). https://doi.org/10.3923/ajft.2007.641.651
W. Dong, R. Hu, Z. Chu, J. Zhao, L. Tan, Effect of different drying techniques on bioactive components, fatty acid composition, and volatile profile of Robusta coffee beans. Food Chem. 234, 121–130 (2017). https://doi.org/10.1016/j.foodchem.2017.04.156
C. Geromel, L.P. Ferreira, F. Davrieux, B. Guyot, F. Ribeyre, M.B. dos Santos Scholz, L.F.P. Pereira et al., Effects of shade on the development and sugar metabolism of coffee (Coffea arabica L.) fruits. Plant Physiol. Biochem. 46(5–6), 569–579 (2008). https://doi.org/10.1016/j.plaphy.2008.02.006
G.V. de melo Pereira, D.P. Carvalho Neto, A.I. Magalhães Júnior, Z.S. Vásquez, A.B.P. Medeiros, L.P.S. Vandenberghe, C.R. Soccol, Exploring the impacts of postharvest processing on the aroma formation of coffee beans—a review. Food Chem. 272, 441–452 (2019). https://doi.org/10.1016/j.foodchem.2018.08.061
M.A. Sfredo, J.R.D. Finzer, J.R. Limaverde, Study of the drying process of Arabica coffee cherries using vibrated trays driers in the fine drink attainment, Harvard. In The 13th International Drying Symposium (IDS 2002), (2002), pp. 1342–1351
M.A. Sfredo, J.R.D. Finzer, J.R. Limaverde, Heat and mass transfer in coffee fruits drying. J. Food Eng. 70(1), 15–25 (2005). https://doi.org/10.1016/j.jfoodeng.2004.09.008
K. Hayat, S. Abbas, C. Jia, S. Xia, X. Zhang, Comparative study on phenolic compounds and antioxidant activity of Feutrell’s Early and Kinnow peel extracts. J. Food Biochem. 35, 454–471 (2011). https://doi.org/10.1111/j.1745-4514.2010.00395.x
H. Noreen, N. Semmar, M. Farman, J.S.O. McCullagh, Measurement of total phenolic content and antioxidant activity of aerial parts of medicinal plant Coronopus didymus. Asian Pac. J. Trop. Med. 10, 792–801 (2017). https://doi.org/10.1016/j.apjtm.2017.07.024
K. Hayat, X. Zhang, U. Farooq, S. Abbas, S. Xia, C. Jia, F. Zhong, J. Zhang, Effect of microwave treatment on phenolic content and antioxidant activity of citrus mandarin pomace. Food Chem. 123, 423–429 (2010). https://doi.org/10.1016/j.foodchem.2010.04.060
M.W. Cheong, K.H. Tong, J.J.M. Ong, S.Q. Liu, P. Curran, B. Yu, Volatile composition and antioxidant capacity of Arabica coffee. Food Res. Int. 51, 388–396 (2013). https://doi.org/10.1016/j.foodres.2012.12.058
I. Hečimović, A. Belščak-Cvitanović, D. Horžić, D. Komes, Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem. 129, 991–1000 (2011). https://doi.org/10.1016/j.foodchem.2011.05.059
S. Roshanak, M. Rahimmalek, S.A.H. Goli, Evaluation of seven different drying treatments in respect to total flavonoid, phenolic, vitamin C content, chlorophyll, antioxidant activity and color of green tea (Camellia sinensis or C. assamica) leaves. J. Food Sci. Technol. 53, 721–729 (2016). https://doi.org/10.1007/s13197-015-2030-x
H.S. Kwak, S. Ji, Y. Jeong, The effect of air flow in coffee roasting for antioxidant activity and total polyphenol content. Food Control 71, 210–216 (2017)
M. Doğan, D. Aslan, V. Gürmeriç, A. Özgür, M.G. Saraç, Powder caking and cohesion behaviours of coffee powders as affected by roasting and particle sizes: principal component analyses (PCA) for flow and bioactive properties. Powder Technol. 344, 222–232 (2019)
D. Arslan, M.M. Özcan, Study the effect of sun, oven and microwave drying on quality of onion slices. LWT Food Sci. Technol. 43, 1121–1127 (2010). https://doi.org/10.1016/j.lwt.2010.02.019
C.-H. Chang, H.-Y. Lin, C.-Y. Chang, Y.-C. Liu, Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. J. Food Eng. 77(3), 478–485 (2006). https://doi.org/10.1016/j.jfoodeng.2005.06.061
K. Hayat, X. Zhang, H. Chen, S. Xia, C. Jia, F. Zhong, Liberation and separation of phenolic compounds from citrus mandarin peels by microwave heating and its effect on antioxidant activity. Sep. Purif. Technol. 73, 371–376 (2010). https://doi.org/10.1016/j.seppur.2010.04.026
K. Hayat, S. Abbas, S. Hussain, S.A. Shahzad, M.U. Tahir, Effect of microwave and conventional oven heating on phenolic constituents, fatty acids, minerals and antioxidant potential of fennel seed. Ind. Crops Prod. 140, 111610 (2019)
M.B. Hossain, C. Barry-Ryan, A.B. Martin-Diana, N.P. Brunton, Effect of drying method on the antioxidant capacity of six Lamiaceae herbs. Food Chem. 123, 85–91 (2010). https://doi.org/10.1016/j.foodchem.2010.04.003
É.R. Oliveira, R.F. Silva, P.R. Santos, F. Queiroz, Potential of alternative solvents to extract biologically active compounds from green coffee beans and its residue from the oil industry. Food Bioprod. Process. 115, 47–58 (2019). https://doi.org/10.1016/j.fbp.2019.02.005
G.G. Marcheafave, C.D. Tormena, E.D. Pauli, M. Rakocevic, R.E. Bruns, I.S. Scarminio, Experimental mixture design solvent effects on pigment extraction and antioxidant activity from Coffea arabica L. leaves. Microchem. J. 146, 713–721 (2019). https://doi.org/10.1016/j.microc.2019.01.073
R.A. Dixon, N.L. Paiva, Stress-induced phenylpropanoid metabolism. Plant Cell 7(7), 1085–1097 (1995)
M. Geremu, Y.B. Tola, A. Sualeh, Extraction and determination of total polyphenols and antioxidant capacity of red coffee (Coffea arabica L.) pulp of wet processing plants. Chem. Biol. Technol. Agric. 3(1), 25 (2016). https://doi.org/10.1186/s40538-016-0077-1
N.F. Lasano, A. Rahmat, N.S. Ramli, M.F. Abu Bakar, Effect of oven and microwave drying on polyphenols content and antioxidant capacity of herbal tea from Strobilanthes crispus leaves. Asian J. Pharm. Clin. Res. 11(6), 363–368 (2018). https://doi.org/10.22159/ajpcr.2018.v11i6.24660
S.-N. Lou, Y.-C. Lai, J.-D. Huang, C.-T. Ho, L.-H.A. Ferng, Y.-C. Chang, Drying effect on flavonoid composition and antioxidant activity of immature kumquat. Food Chem. 171, 356–363 (2015). https://doi.org/10.1016/j.foodchem.2014.08.119
J. Pinela, L. Barros, M. Dueñas, A.M. Carvalho, C. Santos-Buelga, I.C.F.R. Ferreira, Antioxidant activity, ascorbic acid, phenolic compounds and sugars of wild and commercial Tuberaria lignosa samples: effects of drying and oral preparation methods. Food Chem. 135, 1028–1035 (2012). https://doi.org/10.1016/j.foodchem.2012.05.038
R. Mohd Salleh, S.Y. Lai, Effects of drying, fermented and unfermented tea of Ocimum tenuiflorum Linn. on the antioxidant capacity. Int. Food Res. J. 20, 1601–1608 (2013)
A.K. Jaiswal, G. Rajauria, N. Abu-Ghannam, S. Gupta, Effect of different solvents on polyphenolic content, antioxidant capacity and antibacterial activity of Irish york cabbage. J. Food Biochem. 36, 344–358 (2012). https://doi.org/10.1111/j.1745-4514.2011.00545.x
V.A. Mirón-Mérida, J. Yáñez-Fernández, B. Montañez-Barragán, B.E. Barragán Huerta, Valorization of coffee parchment waste (Coffea arabica) as a source of caffeine and phenolic compounds in antifungal gellan gum films. LWT Food Sci. Technol. 101, 167–174 (2019). https://doi.org/10.1016/j.lwt.2018.11.013
A. Belay, K. Ture, M. Redi, A. Asfaw, Measurement of caffeine in coffee beans with UV/vis spectrometer. Food Chem. 108(1), 310–315 (2008). https://doi.org/10.1016/j.foodchem.2007.10.024
A. Farah, T. de Paulis, L.C. Trugo, P.R. Martin, Effect of roasting on the formation of chlorogenic acid lactones in coffee. J. Agric. Food Chem. 53(5), 1505–1513 (2005). https://doi.org/10.1021/jf048701t
R.C. Borrelli, F. Esposito, A. Napolitano, A. Ritieni, V. Fogliano, Characterization of a new potential functional ingredient: coffee silverskin. J. Agric. Food Chem. 52(5), 1338–1343 (2004). https://doi.org/10.1021/jf034974x
P.S. Murthy, M.M. Naidu, Recovery of phenolic antioxidants and functional compounds from coffee industry by-products. Food Bioprocess Technol. 5(3), 897–903 (2012). https://doi.org/10.1007/s11947-010-0363-z
S.I. Mussatto, E.M.S. Machado, S. Martins, J.A. Teixeira, Production, composition, and application of coffee and its industrial residues. Food Bioprocess Technol. 4(5), 661–672 (2011). https://doi.org/10.1007/s11947-011-0565-z
L. Regazzoni, F. Saligari, C. Marinello, G. Rossoni, G. Aldini, M. Carini, M. Orioli, Coffee silver skin as a source of polyphenols: high resolution mass spectrometric profiling of components and antioxidant activity. J. Funct. Foods 20, 472–485 (2016). https://doi.org/10.1016/j.jff.2015.11.027
Acknowledgements
The Authors extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for supporting the work through College of Food and Agricultural Sciences Research Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alkaltham, M.S., Salamatullah, A. & Hayat, K. Determination of coffee fruit antioxidants cultivated in Saudi Arabia under different drying conditions. Food Measure 14, 1306–1313 (2020). https://doi.org/10.1007/s11694-020-00378-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00378-4