Abstract
The European common lizard, Zootoca vivipara, is the most widespread terrestrial reptile in the world. It occupies almost the entire Northern Eurasia and includes four viviparous and two oviparous lineages. We analysed how female snout-vent length (SVL), clutch size (CS), hatchling mass (HM), and relative clutch mass (RCM) is associated with the reproductive mode and climate throughout the species range and across the evolutionary lineages within Z. vivipara. The studied variables were scored for 1,280 females and over 3,000 hatchlings from 44 geographically distinct study samples. Across the species range, SVL of reproductive females tends to decrease in less continental climates, whereas CS corrected for female SVL and RCM tend to decrease in climates with cool summer. Both relationships are likely to indicate direct phenotypic responses to climate. For viviparous lineages, the pattern of co-variation between female SVL, CS and HM among populations is similar to that between individual females within populations. Consistent with the hypothesis that female reproductive output is constrained by her body volume, the oviparous clade with shortest retention of eggs in utero showed highest HM, the oviparous clade with longer egg retention showed lower HM, and clades with the longest egg retention (viviparous forms) had lowest HM. Viviparous populations exhibited distinctly lower HM than the other European lacertids of similar female SVL, many of them also displaying unusually high RCM. This pattern is consistent with Winkler and Wallin’s model predicting a negative evolutionary link between the total reproductive investment and allocation to individual offspring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quantity and quality of the offspring a female has produced determines her evolutionary fitness and ultimately the viability of the population in general. In many animal species, particularly in ectotherms, reproductive traits are strongly influenced by maternal body size. Adult body size is also important in many biological contexts other than reproduction (e.g., Blanckenhorn 2000; Meiri 2008). Therefore, offspring body size, offspring number, and body size of reproducing females are primary targets of evolutionary and ecological studies (Stearns 1992; Roff 1992).
Reptiles, particularly lizards exhibit a pronounced variation in reproductive traits and body size within and among species, and in recent decades they have been among the model groups for studying the evolution of reproductive strategies (Vitt and Pianka 1994; Shine 2005). Maternal size, climate, and ancestry (phylogenetic history) were identified as important predictors of variation in reptilian reproductive traits across related species and/or within species (e.g., Bauwens and Díaz-Uriarte 1997; Brandt and Navas 2011; Díaz et al. 2012). Theory and some empirical studies (e.g., Qualls and Shine 1995; Sun et al. 2012) suggest that reproductive mode (egg-laying vs. live-bearing) can also influence life-history traits in reptiles.
Intraspecific variation is an important issue because it links macroevolutionary patterns to microevolutionary processes which lead to the phenotypic diversity we wish to understand. While the role of ancestry is expected to be minor at this level (but see Ashton 2004), a central problem is to estimate whether the observed differences is a purely phenotypic response (plasticity) or a response to local selection (adaptation). Although common-garden and transplant experiments allow disentangling environmental and population-specific sources of geographic variation (Ferguson and Talent 1993; Sorci and Clobert 1999; Lorenzon et al. 2001), such studies are quite laborious and usually involve only two populations. Therefore, when a predominant impact of plasticity or adaptation is revealed, the ‘regular factor’ effect remains confounded with the site effect (Massot et al. 1992; Madsen and Shine 1993; Díaz et al. 2007). Thus, it remains unclear whether the study factor (e.g., a climatic variable) is a major determinant of the variation across the species range.
Wide-ranging species present promising models for simultaneously evaluating the role of different factors shaping the phenotypic diversity, because the variation of target traits can be documented from numerous localities exhibiting diverse combinations of putative predictors. However, comprehensive range-wide studies of geographic variation in widespread species are rare, even for fundamentally important traits such as offspring size and fecundity. Specifically for reptiles, only a few studies on the intraspecific variation of reproductive traits involve multiple populations and cover large geographic areas (Forsman and Shine 1995; Gregory and Larsen 1993; Iverson et al. 1997; Díaz et al. 2012).
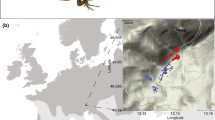
A particularly promising candidate for such a study is the European common lizard, Zootoca vivipara. This species occupies much of the temperate Palaearctic (Fig. 1) and possesses the largest range among terrestrial reptiles. Moreover, Z. vivipara is one of the very few reptile species that occurs in both viviparous and oviparous forms (Braña and Bea 1987). Recent studies have provided an intraspecific phylogeny (Surget-Groba et al. 2006) and detailed life-history data for selected populations (e.g., Heulin 1985; Bauwens and Verheyen 1987; Massot et al. 1992). Surprisingly, no reproductive or life-history traits of this species have been studied range-wide, although some descriptive data for the eastern part of the species range have been provided (Orlova et al. 2005).
The present study aimed to analyse patterns of intraspecific variation in reproductive traits and body size of reproducing females of Z. vivipara throughout the entire species range. Specifically, we assessed the effects of reproductive mode, lineage, and two climatic variables on maternal size, offspring size, and fecundity. We also explored co-variation between these life-history variables among and within populations. Our particular expectations for phenotypic responses to the listed predictors and for the pattern of co-variation are summarized on Fig. 2 and presented in detail below.
Putative relationships among dependent and independent variables, and predictions tested in our study. Note that female body size is both a dependent variable and a predictor for reproductive traits. Predictions: P1a, smaller hatchling mass in viviparous versus oviparous populations; P1b, smaller clutch size in viviparous versus oviparous populations; P2, larger relative clutch mass in viviparous versus oviparous populations; P3, larger female body length in viviparous versus oviparous populations; P4, larger female body length in populations experiencing shorter versus longer activity season; P5, smaller clutch size (a) and clutch mass (b) relative to female body size in populations experiencing colder versus warmer climate; P6, larger hatchling mass in populations experiencing colder versus warmer climate; P7 (not shown), structure of co-variation between offspring size, offspring number and female body length among population means is similar to the pattern among individual females within populations
Our major prediction was that live-bearing populations of Z. vivipara do not show considerable evolutionary divergence in life-history. First, this hypothesis is most parsimonious. Second, extensive experimental studies of life-history variation in Z. vivipara revealed predominantly plastic responses to variation in physical environments (Sorci et al. 1996; Sorci and Clobert 1999; Lorenzon et al. 2001). Generally, a comparative study cannot differentiate between genetic adaptation and phenotypic plasticity. Yet in lizard life-history, for several among-trait and trait-climate relationships the prevailing adaptive hypothesis and the prevailing plasticity-related hypothesis predict opposite patterns. Differences in reproductive strategies between viviparous and oviparous strains are predicted to be adaptive evolutionary response to physical constraints due to retention of eggs in utero.
Reproductive Mode
The phylogenetic transition from oviparity to viviparity (i.e. progressive retention of eggs in utero throughout embryogenesis) offers an opportunity to test several predictions of life-history theory, in particular the hypothesis that female reproductive output is constrained by abdomen volume (Qualls and Shine 1995; Du et al. 2005). At later stages of the embryo development, the eggs increase substantially in their mass and volume due primarily to the uptake of water (Guillette 1982; Qualls and Shine 1995; Sun et al. 2012; and references therein). In typical oviparous species, this swelling occurs after oviposition and does not burden the female. In contrast, the viviparous female retains the swelling eggs in her oviducts until the end of their development. Thus the viviparous female not only carries her reproductive burden longer than the oviparous female, but this burden, by the same net offspring mass, is larger. As a consequence, viviparous populations are expected to show either reduced offspring size (Prediction 1a) and/or number (Prediction 1b) to balance the burden, or increased relative clutch mass (Prediction 2) to maintain the net reproductive output, or increased female size (Prediction 3). These predicted responses do not exclude one another, but each of them may have fitness costs. Only few studies compared life-history traits in conspecific populations (Qualls and Shine 1995; Smith and Shine 1997; Lindtke et al. 2010) or related species (Guillette 1982; Shine 1987; Medina and Ibargüengoytía 2010; Sun et al. 2012; Yang et al. 2012) which differ in reproductive mode. Their results showed no clearly consistent pattern. Z. vivipara is particularly suitable for studying life-history correlates of the evolution towards viviparity because in this species each reproductive mode is represented with at least two non-sister lineages (clades) (Surget-Groba et al. 2006) thus allowing us to disentangle the effect of reproductive mode from that of phylogeny. Moreover, the two oviparous clades exhibit different degrees of egg retention (Heulin et al. 2002). Therefore, testing the above predictions, three (rather than merely two) stages along the oviparity-viviparity axis can be compared for the target traits.
Climate
As the maternal body size is often the major determinant of geographic variation in clutch size and sometimes offspring size (e.g., Vitt and Breitenbach 1993; Iverson et al. 1997; Kiefer et al. 2008; Díaz et al. 2012), climate can act by affecting the body size distribution of reproducing females (Fitch 1985; Shine 2005; Angilletta et al. 2006). Adolph and Porter (1996) modeled the interaction of lizard growth and maturation patterns in seasonal climates. Their model predicts a nonlinear relationship between the length of activity season and the age at the first reproduction which consequently affects the minimum and average body size of adults. In the year of life in which the warm-climate individuals enter the reproduction, the individuals from colder environments (or other environments which shorten the activity season) cannot reach an appropriate body size within the time of the season at which the reproduction is still suitable. Therefore, they invest available energy to further growth and enter reproduction in the following year but at a larger size. The emerging body size cline corresponds to Bergmann’s rule (larger body size in colder climates: Blackburn et al. 1999), but the underlying mechanism is a direct response to environmental constraint rather than genetic divergence (Adolph and Porter 1996). In Z. vivipara, a marginally significant Bergmann’s cline was reported for altitudinal variation within an oviparous clade in northern Spain (Rodríguez-Díaz and Braña 2012). We tested whether this trend is consistent across the whole range (Prediction 4). Although Bergmann’s clines may be adaptive under some conditions (Adolph and Porter 1996; Angilletta et al. 2004), the existence of a plausible explanation of this pattern as a direct response to climate (Adolph and Porter 1996) is an important point here. Note that for the opposite pattern (converse-Bergmann’s clines), only an adaptive explanation regarding the squamate reptiles (small-sized organisms gain heat more rapidly, and in cool environments they can use short periods of sunny time more effectively than large-bodied individuals) has been proposed (Ashton and Feldman 2003; Pincheira-Donoso et al. 2008).
Climate can also affect reproductive traits independently of female body size. Female size-adjusted reproductive output is predicted to decrease in colder climates as a direct response to reduced activity and energy acquisition opportunities under thermal constraints (Congdon 1989; Adolph and Porter 1993; Rohr 1997). We tested if clutch size and clutch mass relative to female body size decreases in colder climates (Predictions 5a and 5b) in Z. vivipara, the species which expands further to the north than any other reptile. Noteworthy, in lizards in general the opposite pattern, an increase of clutch size in colder climates, is obviously predominant (Fitch 1985; Taylor et al. 1992; Vitt and Breitenbach 1993; Rocha et al. 2002; references in these papers). Although the latter pattern was found in two viviparous species (Wapstra and Swain 2001; Rocha et al. 2002), the vast majority of the species in those studies are oviparous that often produce multiple clutches in warmer climates. Consequently, the increased clutch size in colder climates can be viewed as an adaptive evolutionary response to reduced clutch frequency (Cox et al. 2003). For some special cases a plasticity-related explanation of the latter eco-geographic trend is also possible. In arid areas, deficiency of water and, consequently, low productivity may constrain energy acquisition at lower elevations more strongly than at higher elevations, with “colder” sites actually providing more benign environment than “warmer” sites (Brandt and Navas 2011; Díaz et al. 2012). However, like for converse-Bergmann’s clines, no broadly applying hypothesis viewing increased clutch size as a direct response to colder climates has been erected.
The available evidence for direct effects of climatic conditions on offspring size does not seem to allow plausible predictions regarding geographic variation. For Z. vivipara, it has been shown that females experiencing more rainfall during gestation produce smaller hatchlings, but their daughters subsequently produce fewer but larger hatchlings (Marquis et al. 2008). In contrast, there is a well established adaptive hypothesis which predicts a shift to a reproductive strategy with fewer and larger offspring in harsh environments constraining juvenile growth (Parker and Begon 1986; Roff 1992; Johnston and Leggett 2002). In Z. vivipara, hatchling size in the northern and southern populations of Sweden did differ as predicted by the above theory (Uller and Olsson 2003), and we examined whether this pattern is consistent across the species range (Prediction 6).
Co-variation of Offspring Size, Offspring Number, and Maternal Size
We compared the pattern of correlation structure between offspring size, offspring number, and maternal size among individual females within populations with that among populations. The within-population correlations tend to roughly correspond to genetic correlations, the latter being maintained by stabilizing selection to favor phenotypic integration (e.g., Arnold and Phillips 1999). Similarity between the within- and among-population correlation structure (Prediction 7) is expected if genetic differentiation between study populations is negligible or largely due to stochastic processes (Revell et al. 2007 and references therein). Directional selection can break the within-population correlations, however, so that considerable adaptive divergence may often be associated with substantial differences between the within- and among population patterns (cf. Merilä and Björklund 1999; Revell et al. 2007). Thus, the above comparison provides additional indirect test of our major hypothesis.
Materials and Methods
Study Species
Zootoca vivipara is a small (adult snout-vent length 41–77 mm), ground-dwelling, insectivorous, heliothermic lizard. It occupies nearly the whole Europe and much of the northern Asia. Compared to most other lizards Z. vivipara shows a high resistance to low temperatures and a low resistance to desiccation (Reichling 1957). It prefers humid habitats, mostly in the forest vegetation zone.
Zootoca vivipara exhibits reproductive bimodality, with both the oviparous and the viviparous mode being represented with several distinct clades (lineages): western oviparous clade (WO) occupies Cantabrian Mountains and the Pyrenees with adjacent lowland areas; the Eastern Oviparous clade (EO) occurs in northern Italy, Slovenia, and southern Austria; Western Viviparous clade (WV) inhabits nearly the whole Western Europe from France to western Scandinavia, West Carpathians, and a part of Balkans; and Eastern Viviparous clade (EV) covers the greatest, eastern part of the species range (Fig. 1); two further, apparently relic clades, Central Viviparous I and Central Viviparous II, were identified from the south of central Europe (Surget-Groba et al. 2006). Their relationships derived from mitochondrial-DNA polymorphisms (Surget-Groba et al. 2006) are shown in Fig. 1b. This pattern, particularly the basal position of EO clade and a clearly distant relatedness between the two oviparous strains, is in accordance with the karyotype and chromosome structure variation (see Arribas 2009 and Lindtke et al. 2010 for brief reviews).
The young of viviparous females are usually born in the membranes and hatch within 1–2 h after oviposition (e.g., Lorenzon et al. 2001; Vercken and Clobert 2008). Less frequently, young go out of the membrane before parturition (e.g., Kuranova and Yartsev 2012), and hatching 4–6 days after parturition was also reported (Eplanova 2009; Lindtke et al. 2010).
Eggs laid by the oviparous Z. vivipara females possess true eggshell but contain embryos at later developmental stages than the freshly laid eggs of most other lacertid lizard species and have a relatively short incubation period of 26–37 days (Braña et al. 1991; Heulin et al. 2002; Lindtke et al. 2010; Rodríguez-Díaz and Braña 2012).
For simplicity, and taking into account the lack of qualitative distinction between the two reproductive modes in the study species, we hereafter apply terms “oviposition”, “clutch”, and “hatchling” to all populations (instead of “parturition”, “litter”, and “newborn/neonate” usually applied for viviparous taxa).
Samples and Characters
We summarized original and published data on the following traits: snout-vent length of reproducing females, SVL; clutch size (number of eggs per clutch), CS; mean hatchling mass per clutch, HM; post-partum female mass, PPM; clutch mass, CM, taken as a difference between the female mass shortly before oviposition and the post-partum female mass in studies on viviparous populations (Pilorge et al. 1983; Pilorge 1987; Bauwens and Verheyen 1987; this study), and as total mass of freshly laid eggs in studies on oviparous populations (Braña et al. 1991; Osenegg 1995); relative clutch mass, RCM = CM/PPM.
SVL is the primary measure of overall body size in lizards and snakes (Roitberg et al. 2011 and references therein). Body mass is generally less suitable for comparative studies as it typically varies with reproductive status, fat storage, digestive state, and state of the tail. However, just PPM (except females which miss a large part of the tail) and particularly HM are free from these faults. Moreover, HM is clearly a more suitable estimator of offspring size than SVL, because inaccuracy of body length measurement in tiny newborns is too large relative to true natural variability (Massot et al. 1992). This problem is particularly relevant to our study because the data come from different researchers and, hence, may additionally include inter-observer bias (Roitberg et al. 2011).
CS reflects the fecundity in a single reproductive bout. It is an appropriate metric for total fecundity because in the vast majority of the common lizard populations (all viviparous and the highland oviparous) females produce only one clutch per year.
The RCM metric is widely used as measure of reproductive investment in reptiles (e.g., Shine 1992) because most of them, including Z. vivipara, display no parental care after egg laying. RCM also estimates the physical burden the female carries out (Qualls and Shine 1995).
All data on body mass and a larger part of the clutch size data have been obtained via monitoring of gravid females which were caught from the wild and held in captivity for a few days or weeks under standard conditions (Pilorge 1987, with minor modifications in other studies e.g. Uller and Olsson 2003; Lindtke et al. 2010) until parturition. The post-partum female mass and the mass of viable offspring were measured within 24 h after parturition. The female mass before parturition was recorded within the last 1–3 days of pregnancy. As manipulating of the near-term females is potentially stressful for them or their offspring, the latter trait was recorded in a smaller fraction of the females in our own studies.
A part of the clutch size data was obtained by counting oviductal eggs, embryos or (a minor fraction of data) follicles in terminal phases of vitellogenesis on autopsied females, along with measuring maternal SVL. These data come from museum samples or from previous studies mostly performed for parasitological monitoring (Kuranova et al. 2011). No animals have been sacrificed for the present study.
Additional individual-based data on the CS and maternal SVL were extracted from published scatterplots (e.g., Pilorge et al. 1983) or tables (Juszczyk 1974). Furthermore, we implemented in our analyses published mean values and other summary statistics for the relevant characters. We applied the following inclusion criteria for data from literature: (1) geographic origin of the study sample is clearly defined; (2) the sample size and some metric for variation (SD, SE or at least extreme values) are given, along with mean values; (3) data for at least two study traits are available for a given sample.
Since non-viable offspring tend to be lighter than the viable hatchlings (Massot et al. 1992) these were excluded from computations of hatchling mass. We only considered non-viable offspring when estimating clutch size to avoid a bias with the data coming from autopsied females. Deducing from extensive clutch size data on a Z. vivipara population in southern France (Eizaguirre et al. 2007), exclusion of non-viable offspring would result in quite minor decrease (0.07–0.20) of mean values; this bias is negligible as compared to the studied geographic variation (e.g., Orlova et al. 2005).
In total, data from 1,280 females/clutches and over 3,000 hatchlings from 76 localities across the species range were summarized (“Appendix”).
Climatic data (monthly mean minimum and maximum temperatures, and monthly mean precipitation) for the 76 study localities were obtained through the WorldClim database version 1.4, which is based on weather conditions recorded from 1950 to 2000. The spatial resolution is approximately 900 m × 900 m for Central Europe and somewhat lower for the other Eurasian regions (Hijmans et al. 2005). For few sites located in mountain regions the altitude value provided by the WorldClim deviate substantially (200–500 m) from the value given for the corresponding study sample in the original citation (apparently due to a bias in the reported coordinate values or local faults within the data base). In these cases, we corrected all temperatures for adiabatic cooling/heating (lapse rate = 0.65 °C/100 m), following the approach of Angilletta et al. (2006).
Data Analysis
Whenever reasonable sample sizes (n > 10 females/clutches) were available we used strictly local samples, both for original and published data. Within localities, samples from different years were pooled to increase sample sizes and to apply a standard approach across the data. When local sample sizes were too small, however, we pooled them into compound samples (Fig. 1) and used in our analyses weighted means for the study traits and unweighted means for climatic variables. By pooling samples we considered (1) geographic distances, especially relative to the adjacent study samples; (2) homogeneity in terms of altitude, plant community zone, and climate; (3) lack of pronounced differences between samples for the studied traits. In few cases, data of smaller sample sizes were included if no other datasets for particular geographic region were available.
To avoid the problem of non-independence of data collected from siblings, the mean value for each clutch was used to analyze variation in hatchling mass in this and most other studies (e.g., Massot et al. 1992; Uller and Olsson 2003; Lindtke et al. 2010). Taking into account, however, that considering each hatchling as an independent observation did not significantly bias the results (Sorci and Clobert 1999; this study), population means derived with this approach were also included in our geographic variation analyses.
Considering the critique of using ratios (e.g., Packard and Boardman 1988) and regression residuals (e.g., Garcia-Berthou 2001) we controlled for confounding effects of correlated traits using ANCOVA (by placing these variables as covariates) whenever possible. We only used regression residuals to visualize a relationship of clutch size adjusted to female SVL with a climatic variable (Fig. 4) and to examine whether the revealed relationship is confounded by spatial autocorrelation. We also used a logarithm of the ratio RCM as a single dependent variable, because for many study samples the numerator (CM) or the denominator (female postpartum mass) values were not available. A lack of significant correlation between RCM and female body size in virtually all studies which tested this relationship in many lizard species (e.g., Marco et al. 1994; Qualls and Shine 1995; Olsson and Shine 1997; Tinkle et al. 1993; Wapstra and Swain 2001; Chamaillé-Jammes et al. 2006; Stuart-Smith et al. 2007; but see Pilorge et al. 1983) could argue for relevance of this widely used character, at least for rough estimations. Noteworthy, the logarithm of another ratio of two correlated traits, population means of male size and female size, was shown to have reasonable statistical properties and suggested as an appropriate metric for sexual size dimorphism (Smith 1999).
To simultaneously analyze categorical effects (Reproductive mode, Clade nested within Reproductive mode) and continuous effects (two climatic vectors and/or phenotypic variables, see below) on the variation among population means of a target trait we used generalized linear models with normal distribution and identity-link and selected the best model using Akaike’s Information Criterion for finite samples (AICc). The best model was then rerun as general linear model to assess the overall and relative strength of different predictors.
For female body length (SVL) which we treated as independent from the reproductive variables, only climatic vectors were used as covariates. For CS the set of covariates was extended for female SVL because the CS—female SVL relationship is strong and consistent in this species (e.g., Pilorge et al. 1983; this study). For HM, the co-variation with female SVL is not that strong and consistent in lizards as the CS—female SVL relationship. However, just in Z. vivipara, a significant interrelationship of HM, CS, and female SVL within populations was revealed (Massot et al. 1992; this study). This pattern was also revealed in variation among confamiliar species (Bauwens and Díaz-Uriarte 1997). Thus for HM the original set of covariates included two climatic and two phenotypic variables.
To investigate the possibility that an effect of climate on a study trait inferred from above models is an artefact of isolation by distance, we next tested for a relationship between among-sample distances for a study trait, climatic vectors and geographical distances. The statistical correlation between matrices of phenetic, climatic, and geographic distances was evaluated using simple and partial Mantel tests. The software used was zt (Bonnet and Van de Peer 2002), the number of permutations was 10,000. As sample 44 was a clear outlier in terms of the geographic distances (Fig. 1), it was excluded from our Mantel tests.
A principal components analysis (PCA) was used to explore patterns of multivariate divergence among populations, and of the variability among individual females/clutches within populations, in the three basic life-history variables—offspring body size (HM), offspring number (CS), and maternal body size (SVL). Principal components were extracted from the matrix of correlations among sample means (“Appendix”) and the matrix of pooled within-group (among individual females) correlations. The latter data set included seven study samples (in total 161 females) in which the three traits were recorded on at least ten females. For among-sample variation, a separate PCA was run for (1) all samples in which the three traits were available, (2) all except the single sample of the EO clade which exhibits a distinctly high hatchling mass, and (3) for the two viviparous clades. Analysis 2 was performed to test the robustness of component structure of Analysis 1; Analysis 3 can be particularly relevant for comparing with the within-sample variation because the latter was estimated on samples belonging to just these two clades.
PCA was also used to summarize the geographic variation of climatic parameters: the 36 inter-correlated temperature and precipitation variables were reduced to a smaller set of orthogonal vectors which include a major portion of the total variation. These principal components were then used as independent variables in the partial correlation and ANCOVA procedures.
When necessary, variables were log-transformed to meet the requirements of parametric tests.
Considering the Effects of Evolutionary Lineage
A phylogeographic study by Surget-Groba et al. (2001, 2006) provided reasonably dense covering of border areas between the major clades. Thereby, virtually all our study samples could be readily assigned to particular clades based on their geographic locations. In samples 6 and 7 which come from a contact area of several clades (Fig. 1a), all study animals were examined for mt-DNA haplotypes (Lindtke et al. 2010; W. Mayer, personal communication). Surget-Groba et al. (2001, 2006) provided phylogenetic relationships among clades (Fig. 1b), but their published data gave really scarce information on the geographical distribution of haplotypes within the clades, and the haplotype variation within clades is apparently little, especially within the EV clade. Therefore, following Díaz et al. (2012), we employed by clade analysis (see above) to partial out the evolutionary pathway effects. Applicability of established comparative phylogenetic procedures for the intraspecific variation, which may well include reticulated evolution, is still debatable (e.g., Stone et al. 2011; see also Díaz et al. 2012); moreover, the number of units in our study is low (four clades, three dichotomies—Fig. 1b).
Methodological Caveats
Numerous factors unrelated to geographic variation, such as short-term fluctuations in food resources or body size distributions of reproducing females, could affect reproductive traits in a particular study sample (Fitch 1985; Shine 2005). Further biases can come from pooling data of several independent researchers. They may differ in measuring routine, type of material (living vs. preserved females), and in collecting and monitoring of gravid females. The biases from the first two factors are expected to be within 2 mm or so (Vervust et al. 2009; Roitberg et al. 2011), and that is much lower than the observed variation within and among our study samples. Animal keeping details should also be a minor caveat for this study because the vast majority of gravid females were obviously collected after ovulation, the stage at which their reproductive output is determined (Bauwens and Verheyen 1987; Uller and Olsson 2005). Finally, and most importantly, the above confounding factors are unlikely to create a regular pattern shaped by a large number of independently collected data units. Such robust patterns are considered for our discussion.
Results
Climatic Variation Across the Study Sites
The first axis of the principal components analysis on the climatological variables (PC1-clim) explained 57.1 % of the total variance among localities (Table 1). PC1-clim is strongly and positively loaded with all monthly temperature and precipitation parameters outside the warmest quater (Table 1); PC1-clim is highly correlated with mean annual temperature (Spearman rank correlation coefficient, p s = 0.98, n = 44, P < 0.001) and mean temperature of the coldest (p s = 0.96, P < 0.001) but not the warmest (p s = 0.27, P > 0.09) quarter.
In contrast, the second principal component (PC2-clim, 27.8 % of the total variance, Table 1) is heavily loaded just with the monthly values of the warmest season (May–August), with consistently positive loadings of temperatures and consistently negative loadings of precipitation (Table 1). PC2-clim is tightly related to the mean temperature of the warmest quarter (p s = 0.94, n = 44, P < 0.001) but not the coldest quarter (p s = 0.17, n = 44, P > 0.3).
Body Length (SVL) of Reproducing Females
For mean SVL of reproducing females, the best model, explaining about 40 % of the among-sample variance, included only one predictor, PC1-clim (Table 2). The relationship between the two variables is shown on Fig. 3. Mantel test revealed a significant correlation between the matrix of among-sample distances for PC1-clim with the matrix of geographic distances (r = 0.613; P = 0.023). When the effects of proximity are factored out, then SVL is still significantly associated with PC1-clim (partial Mantel test, r = 0.295; P < 0.001). Geographic proximity is poorly associated with SVL, however, when the effects of PC1-clim are factored out (partial Mantel test, r = −0.110; P = 0.052).
Mean snout-vent length (SVL) of reproducing females in different study samples of Zootoca vivipara and scores of PC1-clim which encompasses 57 % of the total climatic variation. PC1-clim is highly positively loaded with mean monthly values of minimum temperature, maximum temperature, and precipitation of all months besides the warmest quarter (see Table 1 for details). Numbers of study samples as in Fig. 1 and “Appendix”
Noteworthy, the two most divergent populations of the eastern viviparous clade—“giant” females from the eastern slope of the Kuznetsky Alatau ridge (mean SVL 70 mm, sample 39) and small-sized females from the Lake Markakol coast (mean SVL 54 mm, sample 43)—considerably deviate from the general climate-related trend (Fig. 3). Both populations are located within the Altai–Sayan Mountain Region which lies to the south–east of the West Siberian Plain.
Clutch Size
Mean clutch size showed a considerable geographic variation across the species range (from 4.3 to 9.8—“Appendix”), but our best model (including PC2-clim only) explained less than 12 % of the total variance (Table 2). When the set of predictors had been extended with Female SVL the best model explained above 50 % of the total variance; besides Female SVL this model included PC2-clim and the Female SVL × PC1-clim interaction (Table 2). The relationship between relative CS (taken as residuals of the regression of mean CS on mean female SVL across study samples) and PC2-clim is shown on Fig. 4a.
Mantel test revealed no correlation between the matrix of among-sample distances for relative CS with the matrix of geographic distances (r = 0.03; P = 0.210) so that the above relationship is not an artefact of spatial autocorrelation.
Relative Clutch Mass (RCM)
Mean RCM varies considerably among study samples, ranging from 45 to 49 % in the WO clade, 40 to 82 % in the WV clade, and 48 to 76 % in the EV clade (“Appendix”). The best model, explaining about 50 % of the among-sample variance, included Reproductive mode and PC2-clim (Table 2). When we replaced the two groups of closely located samples (11, 12, 13 and 34, 35, 36—Fig. 1) with the average values we got the same significant predictors with even a higher rate (70 %) of explained variance. The relationship between RCM and PC2-clim is shown on Fig. 4b.
Within the group of viviparous populations, Mantel test revealed no correlation between the matrix of among-sample distances for RCM with the matrix of geographic distances (r = −0.126; P = 0.127), whereas the correlation with PC2-clim was significant both with (r = 0.295; P = 0.029) and without (r = 0.289; P = 0.036) control for geographic proximity. Thus, the revealed relationship between RCM and PC2-clim is not an artefact of spatial autocorrelation.
Mean Hatchling Mass
The best model, explaining 63 % of the total variance, included Reproductive mode and Clade (Table 2). Hatchlings of the single study sample of the EO clade are much heavier than these of all other study populations (Table 3), and hatchlings of the WO clade are heavier than those of the WV clade (ANOVA, the three clades with multiple samples: F 2,19 = 4.43, P < 0.03; Tukey HSD test, P < 0.05; Table 3). Thus our results clearly demonstrate that hatchlings tend to be heavier in oviparous than in viviparous populations, and within the former group hatchlings are heavier in the EO clade.
Within the group of viviparous populations, Mantel test revealed a significant correlation between the matrix of among-sample distances for HM with the matrix of geographic distances (r = 0.168; P = 0.031). This correlation persisted when corrected for PC1-clim (r = 0.211; P = 0.024) or PC2-clim (r = 0.187; P = 0.024).
Co-variation Between Female SVL, Clutch Size, and Hatchling Mass
Principal components analysis (PCA) of within-sample and between-sample variation for female SVL, clutch size and hatchling mass (Table 4) revealed a pronounced similarity in correlation structure at these two levels. This similarity is particularly strong when the data set for geographic variation is restricted to the two viviparous clades (a separate considering of these two clades is relevant because they form a monophyletic unit and because the within-sample variation was estimated on samples belonging to these two clades). In both the within-sample and among-sample variations, PC1 includes 51–59 % of the total variance and is strongly associated with female SVL and clutch size, whereas PC2 (33–34 %) is heavily loaded with hatchling mass and weakly correlated with the two other traits. When the whole data set (including oviparous populations) is considered, the among-sample variation differs from the within-sample variability by a lack of the following tendencies: a positive association between hatchling size and female SVL on PC1 and a negative association between clutch size and hatchling mass on PC2 (Table 4).
Figure 5 shows ordination of study samples along the first two principal components which include ca. 90 % of the considered geographic variation. As expected, the single sample of the EO clade is strongly separated from all other samples along PC2 due to its high hatchling mass (Table 3). Furthermore, the oviparous samples tend to cluster from the viviparous samples by a combination of higher values of PC2 and lower values of PC1. In the subset of viviparous samples, neither clades nor major geographic regions tend to cluster, and these which do cluster (samples 16, 24, and 44) differ strongly in their geographic origin (eastern Germany, Middle Volga, and north-eastern China, respectively).
Discussion
Overall, our study showed a considerable amount of geographic variation in mean female body size and reproductive life-history in Z. vivipara. The study traits differed strongly in the set of their predictors in our analyses. Female SVL and clutch size were affected by climatic vectors, with no significant impact of reproductive mode or evolutionary lineage (clade). These effects remained significant when the effects of geographic proximity were controlled for. In contrast, mean hatchling mass was strongly affected by reproductive mode and clade (and weakly by geographic proximity), without respect to climate. The variation of RCM showed some intermediate pattern, with climate and reproductive mode and/or clade being significant predictors. Below we put the particular trait-predictor relationships into the context of other life-history studies and discuss how they relate to our special predictions (Fig. 2). The difference in the set of predictors is in line with findings from other lizard ife-history studies: in common garden experiments (Ferguson and Talent 1993) and long-term studies of a model population (Massot et al. 1992; Chamaillé-Jammes et al. 2006; Le Galliard et al. 2006), hatchling mass was the least plastic trait and showed large heritability (Le Galliard et al. 2006).
The above patterns for female size and fecundity correspond well with our principal hypothesis on a major role of plasticity in the geographic life-history variation in Z. vivipara. The pattern for offspring size apparently manifests evolutionary divergence between viviparous and oviparous strains. Below we put the particular trait-predictors relationships into the context of other life-history studies and discuss how they relate to our special predictions (Fig. 2).
Reproductive Mode
A decrease of hatchling mass in the series EO clade—WO clade—viviparous populations corresponds well to the hypothesis that female reproductive output is constrained by abdomen volume (Qualls and Shine 1995; Du et al. 2005), and this constraint is exerted by progressive egg retention (Guillette 1982; Qualls and Shine 1995). EO clade occupies the basal position in the species phylogeny (Surget-Groba et al. 2006) and exhibits only a moderate rate of egg retention (Table 3); this clade has the heaviest hatchlings (Table 3). WO clade has a higher rate of egg retention and, respectively, lower hatchling mass (Table 3). Finally, viviparous females have still lighter hatchlings (Table 3). Note that the above pattern of among clade differences in hatchling mass does persist after correcting for variation in female SVL and clutch size, being also resistant to correcting for climate.
Although EO clade is represented by a single population in our data set, a large magnitude of its differences from the other populations (Table 3), including the parapatrically occurring viviparous population (Lindtke et al. 2010), allows us to be confident of the terminal position of EO clade in the above series. Interestingly, mean hatchling mass of EO clade (as estimated with the single study sample), being a positive outlier among the conspecific populations, is yet lower than in the other related species with comparable female SVL for which we could find relevant data (Fig. 6).
Mean hatchling mass and mean body length of reproducing females in different populations of Zootoca vivipara and other European species of the family Lacertidae. Data for Z. vivipara are from “Appendix”; data for other species are from: Arribas and Galán (2005), Castilla and Bauwens (2000), Galán (1999), Galán and Vicente (2003), Ji and Braña (2000), Ljubisavljević et al. (2007, 2012), Rúa and Galán (2003)
Thus, our study clearly demonstrates that in the European common lizard, a progressive extent of egg retention was accompanied by a decrease in offspring size (hatchling mass). Although this pattern of offspring size variation is in line with the ‘volume constraint hypothesis’ (Prediction 1a), this pattern was not common in previous studies. It was reported in none of the other two reproductively bimodal lizard species (Qualls and Shine 1995; Smith and Shine 1997) and in only two of the 7 lizard and snake genera in which oviparous and viviparous species were compared (Guillette 1982; Shine 1987; Medina and Ibargüengoytía 2010; Sun et al. 2012; Yang et al. 2012).
The most frequently reported pattern in the above-cited studies is a larger female size of the viviparous forms versus their oviparous counterparts (both two reproductively bimodal species; 4 of the 7 reproductively bimodal genera—op. cit.). This pattern is also congruent with the ‘volume constraint hypothesis’ (Prediction 3). In Z. vivipara, reproducing females of EO clade show smaller body length than those of WV clade collected from virtually the same site (“Appendix”). Also, populations of WO clade tend to exhibit smaller female SVL than the viviparous populations. However, the smaller female SVL of WO clade appears to be largely induced by milder climate of the region it inhabits (Fig. 3). Furthermore, the existence of a rather large-bodied oviparous population (EO clade, Fig. 3) on the one side, and remarkably small-bodied viviparous populations (samples 15 and 43, Fig. 3) on the other side, argue for a relatively moderate impact of the reproductive mode in shaping the overall geographic variation for female SVL in our study species.
The third pattern related to the ‘volume constraint hypothesis’ is a tendency of viviparous populations to show higher mean RCM than in oviparous populations (Prediction 2). In our study, this pattern is statistically significant when considering for geographic variation in PC2-clim. Considering this circumstance, a lack of data on the EO clade, and the different technique of measuring RCM in oviparous and viviparous females (see “Methods”), we qualify the present evidence as only suggestive. In another reproductively bimodal species, Lerista bougainvillii, mean RCM was clearly higher in the viviparous versus oviparous females (Qualls and Shine 1995), whereas the third species, Saiphos equalis shows an opposite tendency (Smith and Shine 1997). The above-cited between-species comparisons provide no relevant data because they actually used a clearly incomparable metric for RCM based on the total net mass of hatchlings.
Climate
Mantel tests indicated that climate (PC1-clim or PC2-clim) but not geographic proximity, is related to geographic variation in female SVL, relative fecundity, and RCM.
The first principal vector of the inter-locality variation of monthly means of minimal temperature, maximal temperature, and precipitation (PC1-clim, Table 1) can be interpreted as an index of climate mildness. Its negative values correspond to more continental climates with a long winter season and a short (even though sometimes warm) summer, whereas its positive values correspond to benign climates with a short winter and a prolonged period with higher (even though not quite high) temperatures. Therefore, PC1-clim is a good proxy for the length of activity season, and its negative correlation with mean body length (SVL) of reproducing females is in line with Adolph and Porter’s (1996) model. As PC1-clim is tightly associated with mean annual temperature the revealed body size cline corresponds to Bergmann’s rule (Prediction 4). Although Bergmann’s clines were found in many squamate species (e.g. Wapstra and Swain 2001; Rocha et al. 2002 and references therein; Leaché et al. 2010), about 70 % of lizard and snake species exhibit converse-Bergmann’s clines (Ashton and Feldman 2003). Remarkably, even in closely related species, opposite body size clines can occur (Ashton 2001; Sears and Angilletta 2004). In this context it does not seem unreasonable to assume that in a wide-ranging species, multiple independent divergences along similar climatic gradients may differ in their underlying mechanisms. Depending on the relative occurrence of the two patterns in the data set, a trend distorted by outliers (this study) or a lack of an overall trend can be revealed. In any event, the existence of strongly deviating populations in the present study indicates that factors other than the length of activity season (Adolph and Porter 1996) contribute to shaping the geographic body size variation in the study species. Obviously, even the age at maturity is not always the primary determinant of mean body size of reproducing females. In northwestern Europe, mean age at first reproduction is ca. 1.5 years in Paimpont (Heulin 1985), and ≥2 years in Kalmthaut (Bauwens and Verheyen 1987). Opposite to the prediction of Adolph and Porter’s (1996) model the former population exhibits a higher mean SVL of gravid females than the latter one (Fig. 3, Samples 9 and 15, respectively).
The second principal vector of the studied climatic variation (PC2-clim, Fig. 2/Table 1) characterizes the warmest quarter, its lower values being associated with cool and wet summer, and its higher values with warm and relatively dry (even though sometimes short) summer. The positive correlations of PC2-clim with both the SVL-adjusted clutch size and RCM imply that the decrease of relative fecundity in colder climates revealed in Z. vivipara is due to a shift in the total reproductive output rather than in the position of the offspring size-number tradeoff (see also the next paragraph). This pattern, which was also found in another viviparous lizard (Rohr 1997), is consistent with Prediction 5.
We found no significant relationship between offspring size and climate across the main part of the species range occupied by the WV and EV clades. We note, however, that in several cases where two samples from contrasting environments were obtained within the same primary study (samples 4 vs. 5 in French Pyrenees; 24 vs. 25 in the east of European Russia—“Appendix”; southern Sweden vs. northern Sweden—Uller and Olsson 2003), hatchling mass was higher in colder climates. At the same time, hatchlings from females coming from higher vs. lower latitudes in West Siberian plain (samples 30–32 vs. 33–36—“Appendix”), and higher versus lower altitudes in northern Spain (Rodríguez-Díaz and Braña 2012), did not differ in their mass. Thus, the available data on geographic variation of hatchling mass in Z. vivipara provide no unequivocal support for Prediction 6. In lizards in general the predicted pattern is really predominant (Mathies and Andrews 1995; Rohr 1997; Qualls and Shine 2000; Wapstra and Swain 2001; Caley and Schwarzkopf 2004; Du et al. 2010). The opposite pattern, smaller hatchlings at colder climates, has been reported by far less often (Sinervo 1990; Li et al. 2011); moreover, in hot and dry regions, the latter pattern can in fact be consistent with the underlying theoretical models (Parker and Begon 1986; Roff 1992; Johnston and Leggett 2002) because the main constraint on juvenile growth is water deficiency rather than thermal opportunities (Díaz et al. 2012; see also Mateo and Castanet 1994). Noteworthy, for Z. vivipara a higher hatchling size in drier versus more humid habitats located within 10 km around samples 11–13 (Fig. 1) was reported (Lorenzon et al. 2001).
Lineage
Our study revealed significant effects of evolutionary lineage on mean hatchling mass. Whereas no consistent differences were found between the two (sister) viviparous clades, such differences occurred between the viviparous and the oviparous clades, and between WO and EO clades. Although this pattern is concordant to differing extent of egg retention, hatchlings of WO clade are only slightly heavier than those of viviparous populations (Table 3). This small inconsistency may be due to phylogenetic history: oviparity of WO clade is likely a reversal from viviparity (Surget-Groba et al. 2006).
For RCM, the effect of lineage was weak and indistinguishable from the effect of reproductive mode. For female SVL and clutch size, no significant effect of lineage was found when climatic vectors are considerd simultaneously. The above results suggest that the lineage as such (i.e. separated from the effects of the extent of egg retention and climate) have a rather moderate impact to shaping geographic variation of reproductive strategies in Z. vivipara. In another lacertid lizard, Psammodromus algirus, two mt-DNA lineages differed in mean incubation time but not in offspring size, fecundity and maternal size (Díaz et al. 2012). A weak effect of phylogeny on geographic variation of life-history traits was also reported for a widespread iguanian lizard, Sceloporus undulates (Niewiarowski et al. 2004). In contrast, the variation in reproductive and other life-history traits among species of lacertid lizards included a substantial phylogenetic component (Bauwens and Díaz-Uriarte 1997).
Co-variation of Offspring Number, Offspring Size, and Maternal Size
Among viviparous populations the pattern and extent of geographic correlations among hatchling mass, clutch size and maternal SVL is very similar to that among individual females within populations, thus following Prediction 7. That is, despite a huge geographic range, this inter-population variation is unlikely to be associated with a considerable evolutionary divergence. The patterns of covariation within and between populations become less similar when including oviparous samples. This is because the higher hatchling mass of oviparous females versus viviparous females is NOT in line with the slightly positive maternal size-offspring size correlation found within populations (positive factor loadings of the both traits on PC1, Table 4; see also Kuranova and Yartsev 2012). Another study which addressed correlations among reproductive traits in a squamate species revealed a stronger discrepancy between the within-population and among-population patterns, the discrepancy having resulted from a pronounced and obviously adaptive divergence in offspring size (Gregory and Larsen 1993, 1996).
Final Remarks
As was mentioned in the Introduction, for several trait-climate relationships the prevailing adaptive hypothesis and the prevailing plasticity-related hypothesis predict opposite patterns (Table 5). Further, patterns of co-variation among traits within and among populations are expected to be similar when geographic variation is mainly due to plasticity or stochastic genetic processes, but these are likely to differ when a considerable adaptive divergence has occur (cf: Merilä and Björklund 1999; Revell et al. 2007). Even though this association between a pattern and the underlying mechanism is likely rather than strictly obligatory, it is striking that all three independent patterns revealed in Z. vivipara suggest a plasticity-related causation (Table 5). Common garden and transplant experiments on this species (Sorci et al. 1996; Sorci and Clobert 1999; Lorenzon et al. 2001) revealed predominantly plastic responses of life-history traits to variation in physical environments. Taken together, these findings suggest that the reproductive strategy of Z. vivipara generally exhibits evolutionary conservatism, thus inderctly supporting our principal prediction.
The only pattern of considerable evolutionary divergence in reproductive traits revealed in this study is a decrease in offspring size in a series of three population groups (clades) which represent progressive stages of egg retention. The terminal point of this series, viviparous populations, exhibits the lowest hatchling mass among 64 studied species of European lacertids (Table 1 in In den Bosch and Bout 1998; “Appendix” in this paper), including species with clearly smaller female sizes. Note that many viviparous populations also exhibit quite high mean values of relative clutch mass (60–80 %, “Appendix”) which are among the largest in lizards (Van Damme et al. 1989). Such an association of a high total reproductive investment with a low investment per individual offspring is of particular interest. Classical life-history theory (Smith and Fretwell 1974) modeled these two parameters as independent. However, their negative correlation is predicted by a more recent model (Winkler and Wallin 1987), and such an evolutionary link was found in a few empirical studies (Caley et al. 2001 and references therein). Therefore, even though the evolutionary decrease of offspring size in Z vivipara corresponds well to the ‘volume constraint hypothesis’, some additional factors—likely related to an increased selection for fecundity (Einum and Fleming 2000)—might have contributed to this trend. Exploring this complex problem seems to be a promising point for further evolutionary studies on this interesting species.
Another worthy direction of future research is a direct testing of our hypothesis about an evolutionary conservative reproductive strategy in Z. vivipara. Long-term common garden experiments (Ferguson and Talent 1993) involving populations which exhibit contrasting phenotypes could estimate to what extent the observed differences are due to proximate effects of environment. Specifically, the differences in female body size between the two oviparous clades, or among the most divergent viviparous populations (i.e. samples 39 and 43) are interesting targets for such studies.
References
Adolph, S. C., & Porter, W. P. (1993). Temperature, activity, and lizards life histories. American Naturalist, 142, 273–295.
Adolph, S. C., & Porter, W. P. (1996). Growth, seasonality, and lizards life histories. Oikos, 77, 267–278.
Angilletta, M. J., Niewiarowski, P. H., Dunham, A. E., Leaché, A., & Porter, W. P. (2004). Bergmann’s clines in ectotherms: Illustrating a life-historical perspective with sceloporine lizards. American Naturalist, 164, E168–E183.
Angilletta, M. J., Oufiero, C. E., & Leaché, A. D. (2006). Direct and indirect effects of environmental temperature on the evolution of reproductive strategies: An information-theoretic approach. American Naturalist, 168, 123–135.
Anufriev, V. M., & Bobretsov, A. V. (1996). Fauna of the European North-East of Russia, Vol. 4: Amphibians and reptiles. St. Petersburg: Nauka Publ (in Russian).
Arnold, S. J., & Phillips, P. C. (1999). Hierarchical comparison of genetic variance-covariance matrices. II. Coastal-inland divergence in the garter snake, Thamnophis elegans. Evolution, 53, 1516–1527.
Arribas, O. J. (2009). Morphological variability of the Cantabro-Pyrenean populations of Zootoca vivipara (Jacquin, 1787) with description of a new subspecies. Herpetozoa, 21, 123–146.
Arribas, O. J., & Galán, P. (2005). Reproductive characteristics of the Pyrenean high-mountain lizards: Iberolacerta aranica (Arribas, 1993), I. aurelioi (Arribas, 1994) and I. bonnali (Lantz, 1927). Animal Biology, 55, 163–190.
Ashton, K. G. (2001). Body size variation among mainland populations of the western rattlesnake (Crotalus viridis). Evolution, 55, 2523–2533.
Ashton, K. G. (2004). Comparing phylogenetic signal in intraspecific and interspecific body size datasets. Journal of Evolutionary Biology, 17, 1157–1163.
Ashton, K. G., & Feldman, C. R. (2003). Bergmann’s rule in nonavian reptiles: Turtles follow it, lizards and snakes reverse it. Evolution, 57, 1151–1163.
Avery, R. A. (1975). Clutch size and reproductive effort in the lizard Lacerta vivipara Jacquin. Oecologia, 19, 165–170.
Bauwens, D., & Díaz-Uriarte, R. (1997). Covariation of life-history traits in lacertid lizards: A comparative study. American Naturalist, 149, 91–111.
Bauwens, D., & Verheyen, R. F. (1987). Variation of reproductive traits in a population of the lizard Lacerta vivipara. Holarctic Ecology, 10, 120–127.
Blackburn, T. M., Gaston, K. J., & Loder, N. (1999). Geographic gradients in body size: A clarification of Bergmann’s rule. Diversity and Distribution, 5, 165–174.
Blanckenhorn, W. U. (2000). The evolution of body size: What keeps organisms small? Quatary Review of Biology, 75, 385–407.
Bonnet, E., & Van de Peer, Y. (2002). ZT: A software tool for simple and partial Mantel tests. Journal of Statistical Software, 7, 1–12.
Braña, F., & Bea, A. (1987). Bimodalité de reproduction chez Lacerta vivipara. Bulletin de la Société Herpétologique de France, 44, 1–5.
Braña, F., Bea, A., & Arrayago, M. J. (1991). Egg retention in lacertid lizards: Relationships with reproductive ecology and the evolution of viviparity. Herpetologica, 47, 218–226.
Brandt, R., & Navas, C. A. (2011). Life-history evolution on tropidurinae lizards: Influence of lineage, body size and climate. PLoS ONE, 6, e20040.
Caley, M. L., & Schwarzkopf, L. (2004). Complex growth rate evolution in a latitudinally widespread species. Evolution, 58, 862–869.
Caley, M. L., Schwarzkopf, L., & Shine, R. (2001). Does total reproductive effort evolve independently of offspring size? Evolution, 55, 1245–1248.
Castilla, A. M., & Bauwens, D. (2000). Reproductive characteristics of the lacertid lizard Podarcis atrata. Copeia, 2000, 748–756.
Cavin, L. (1993). Structure d’une population subalpine de Lézards vivipares (Lacerta vivipara Jacquin, 1787). Revue Suisse de Zoologie, 100, 357–371.
Chamaillé-Jammes, S., Massot, M., Aragón, P., & Clobert, J. (2006). Global warming and positive fitness response in mountain populations of common lizards Lacerta vivipara. Global Change Biology, 12, 392–402.
Congdon, J. D. (1989). Proximate and evolutionary constraints on energy relations of reptiles. Physiological Zoology, 62, 356–373.
Cox, R. M., Skelly, S. L., & John-Alder, H. B. (2003). A comparative test of adaptive hypotheses for sexual size dimorphism in lizards. Evolution, 57, 1653–1669.
Crnobrnja-Isailović, J., & Aleksić, I. (2004). Clutch size in two Central Balkan populations of European Common Lizard Lacerta vivipara. Biota, 5, 5–10.
Díaz, J. A., Iraeta, P., Verdú-Ricoy, J., Siliceo, I., & Salvador, A. (2012). Intraspecific variation of reproductive traits in a mediterranean lizard: Clutch, population, and lineage effects. Evolutionary Biology, 39, 106–115.
Díaz, J. A., Pérez-Tris, J., Bauwens, D., Pérez-Aranda, D., Carbonell, R., Santos, T., et al. (2007). Reproductive performance of a lacertid lizard at the core and the periphery of the species’ range. Biological Journal of the Linnean Society, 92, 87–96.
Du, W. G., Ji, X., & Shine, R. (2005). Does body-volume constrain reproductive output in lizards? Biology Letters, 1, 98–100.
Du, W. G., Ji, X. A., Zhang, Y. P., Lin, Z. H., & Xu, X. F. (2010). Geographic variation in offspring size of a widespread lizard (Takydromus septentrionalis): Importance of maternal investment. Biological Journal of the Linnean Society, 101, 59–67.
Dufaure, J. P., & Hubert, J. (1961). Table de développement du lézard vivipare: Lacerta (Zootoca) vivipara Jacquin. Archives D’anatomie Microscopique et de Morphologie Expérimentale, 50, 309–328.
Einum, S., & Fleming, I. A. (2000). Highly fecund mothers sacrifice offspring survival to maximise fitness. Nature, 405, 565–567.
Eizaguirre, C., Laloi, D., Massot, M., Richard, M., Federici, P., & Clobert, J. (2007). Condition dependence of reproductive strategy and the benefits of polyandry in a viviparous lizard. Proceedings of the Royal Society, London, Series B, 274, 425–430.
Eplanova, G. V. (2009). Reproductive biology of Zootoca vivipara (Reptilia, Lacertidae) in the Middle Volga Region. Proceedings of Samara Scientific Center, Russian Academy of Sciences, 11, 83–88. (in Russian).
Ferguson, G. W., & Talent, L. G. (1993). Life history traits of the lizards Sceloporus undulatus from two populations raised in a common laboratory environment. Oecologia, 93, 88–94.
Fitch, H. S. (1985). Variation in clutch and litter size in New World reptiles (Vol. 76, pp. 1–76). Lawrence: University of Kansas Museum of Natural History, Miscellaneous Publications.
Forsman, A., & Shine, R. (1995). Parallel geographic variation in body shape and reproductive life history within the Australian scincid lizard Lampropholis delicata. Functional Ecology, 9, 818–828.
Galán, P. (1999). Demography and population dynamics of the lacertid lizard Podarcis bocagei in Northwest Spain. Journal of Zoology London, 249, 203–218.
Galán, P., & Vicente, L. (2003). Reproductive characteristics of the insular lacertid lizard, Teira dugesii. Herpetological Journal, 13, 149–154.
Garcia-Berthou, E. (2001). On the misuse of residuals in ecology: Testing regression residuals vs. the analysis of covariance. Journal of Animal Ecology, 70, 708–711.
Gregory, P. T., & Larsen, K. W. (1993). Geographic variation in reproductive characteristics among Canadian populations of the common garter snake (Thamnophis sirtalis). Copeia, 1993, 946–958.
Gregory, P. T., & Larsen, K. W. (1996). Are there any meaningful correlates of geographic life-history variation in the garter snake, Thamnophis sirtalis. Copeia, 1996, 183–189.
Guillette, L. J., Jr. (1982). The evolution of viviparity and placentation in the high elevation, Mexican lizard Sceloporus aeneus. Herpetologica, 38, 94–103.
Heulin, B. (1985). Démographie d′une population de Lacerta vivipara de basse altitude. Acta Oecologica, Oecologica Generalis, 6, 261–280.
Heulin, B., Ghielmi, S., Vogrin, N., Surget-Groba, Y., & Guillaume, C. P. (2002). Variation in eggshell characteristics and in intra-uterine egg retention between two oviparous clades of the lizard Lacerta vivipara: Insight into the oviparity-viviparity continuum in Squamates. Journal of Morphology, 252, 255–262.
Hijmans, R. J., Guarino, L., Jarvis, A., O’Brien, R., Mathur, P., Bussink, C., et al. (2005). DIVAGIS version 5.2 manual. Available at: www.diva-gis.org.
In den Bosch, H. A. J., & Bout, R. G. (1998). Relationships between maternal size, egg size, clutch size, and hatchling size in European lacertid lizards. Journal of Herpetology, 32, 410–417.
Iverson, J. B., Higgins, H., Sirulnik, A., & Griffiths, C. (1997). Local and geographic variation in the reproductive biology of the snapping turtle (Chelydra serpentina). Herpetologica, 53, 96–117.
Ji, X., & Braña, F. (2000). Among clutch variation in reproductive output and egg size in the wall lizard Podarcis muralis from a lowland population of northern Spain. Journal of Herpetology, 34, 54–60.
Johnston, T. A., & Leggett, W. C. (2002). Maternal and environmental gradients in the egg size of an iteroparous fish. Ecology, 83, 1777–1791.
Juszczyk, W. (1974). Plazy I gady krajowe (p. 722). Warszawa: Panstwowe wydawnictwo naukowe.
Kiefer, M. C., Van Sluys, M., & Rocha, C. F. D. (2008). Clutch and egg size of the tropical lizard Tropidurus torquatus (Tropiduridae) along its geographic range in coastal eastern Brazil. Canadian Journal of Zoology, 86, 1376–1388.
Kuranova, V. N., & Yartsev, V. V. (2012). Some aspects of reproductive biology of the common lizard, Zootoca vivipara. The Problems of Herpetology 142–149. Minsk (in Russian).
Kuranova, V. N., Yartsev, V. V., Kononova, Yu. V., Protopopova, E. V., Konovalova, S. N., Ternovoy, V. A., et al. (2011). Lacertids (Sauria) in natural foci of infections in human-transmitted ecosystems of the south-east of West Siberia. The Problems of Herpetology, 129–135. St. Petersburg (in Russian).
Le Galliard, J. F., Massot, M., Landys, M. M., Meylan, S., & Clobert, J. (2006). Ontogenic sources of variation in sexual size dimorphism in a viviparous lizard. Journal of Evolutionary Biology, 19, 690–704.
Leaché, A. D., Helmer, D., & Moritz, C. (2010). Phenotypic evolution in high elevation populations of western fence lizards (Sceloporus occidentalis) in the Sierra Nevada Mountains. Biological Journal of the Linnean Society, 100, 630–641.
Li, H., Qu, Y. F., Ding, G. H., & Ji, X. (2011). Life-history variation with respect to the experienced thermal environments in the lizard, Eremias multiocellata (Lacertidae). Zoological Science, 28, 332–338.
Lindtke, D., Mayer, W., & Böhme, W. (2010). Identification of a contact zone between oviparous and viviparous common lizards (Zootoca vivipara) in central Europe: reproductive strategies and natural hybridization. Salamandra, 46, 73–82.
Liu, P., Zhao, W. G., Liu, Z. T., Dong, B. J., & Chen, H. (2008). Sexual dimorphism and female reproduction in Lacerta vivipara in northeast China. Asiatic Herpetological Research, 11, 98–104.
Ljubisavljević, K., Glasnović, P., Kalan, K., & Kryštufek, B. (2012). Female reproductive characteristics of the Horvath’s rock lizard from Slovenia. Archives of Biological Sciences, Belgrade, 64, 639–645.
Ljubisavljević, K., Polović, L., Tomašević Kolarov, N., Džukić, G., & Kalezić, M. L. (2007). Female life-history characteristics of the Mosor rock lizard, Dinarolacerta mosorensis (Kolombatović, 1886) from Montenegro (Squamata: Lacertidae). Journal of Natural History, 41, 2979–2993.
Lorenzon, P., Clobert, J., & Massot, M. (2001). The contribution of phenotypic plasticity to adaptation in Lacerta vivipara. Evolution, 55, 392–404.
Madsen, T., & Shine, R. (1993). Phenotypic plasticity in body sizes and sexual size dimorphism in European grass snakes. Evolution, 47, 321–325.
Marco, A., Pérez-Mellado, V., & Gil-Costa, M. J. (1994). Reproductive strategy in a montane population of the lizard Lacerta schreiberi (Sauria, Lacertidae). Herpetological Journal, 4, 49–55.
Marquis, O., Massot, M., & Le Galliard, J. F. (2008). Intergenerational effects of climate generate cohort variation in lizard reproductive performance. Ecology, 89, 2575–2583.
Massot, M., Clobert, J., Pilorge, T., Lecomte, J., & Barbault, R. (1992). Density dependence in the common lizard: demographic consequences of a density manipulation. Ecology, 73, 1742–1756.
Mateo, J. A., & Castanet, J. (1994). Reproductive strategies in three Spanish populations of the Ocellated lizard Lacerta lepida (Sauria, Lacertidae). Acta Oecologia, 15, 215–229.
Mathies, T., & Andrews, R. M. (1995). Thermal and reproductive biology of high and low elevation populations of the lizard Sceloporus scalaris: Implications for the evolution of viviparity. Oecologia, 104, 101–111.
Medina, M., & Ibargüengoytía, N. R. (2010). How do viviparous and oviparous lizards reproduce in Patagonia? A comparative study of three species of Liolaemus. Journal of Arid Environments, 74, 1024–1032.
Meiri, S. (2008). Evolution and ecology of lizard body sizes. Global Ecology and Biogeography, 17, 724–734.
Merilä, J., & Björklund, M. (1999). Population divergence and morphometric integration in the greenfinch (Carduelis chloris)—Evolution against the trajectory of least resistance? Journal of Evolutionary Biology, 12, 103–112.
Niewiarowski, P. H., Angilletta, M. J., & Leaché, A. D. (2004). Phylogenetic comparative analysis of life-history variation among populations of the lizard Sceloporus undulatus: An example and prognosis. Evolution, 58, 619–633.
Olsson, M., & Shine, R. (1997). The limits to reproductive output: offspring size versus number in the sand lizard (Lacerta agilis). American Naturalist, 149, 179–188.
Orlova, V. F., Kuranova, V. N., & Bulakhova, N. A. (2005). Some aspects of reproductive biology of Zootoca vivipara (Jacquin, 1787) in the Asian part of its area. In N. Ananjeva & O. Tsinenko (Eds.), Herpetologia petropolitana (pp. 255–258). St. Petersburg: Societas Europaea Herpetologica.
Osenegg, K. (1995). Populationsökologische Untersuchungen an der oviparen Form der Waldeidechse, Lacerta (Zootoca) vivipara Jacquin, 1787, im Südwesten Frankreichs. Ph. D. Thesis. Bonn University.
Packard, G. C., & Boardman, T. J. (1988). The misuse of ratios, indices, and percentages in ecophysiological research. Physiological Zoology, 61, 1–9.
Parker, G. A., & Begon, M. (1986). Optimal egg size and clutch size: Effects of environment and maternal phenotype. American Naturalist, 128, 573–592.
Pilorge, T. (1987). Density, size structure, and reproductive characteristics of three populations of Lacerta vivipara (Sauria: Lacertidae). Herpetologica, 43, 345–356.
Pilorge, T., Xavier, F., & Barbault, R. (1983). Variations in litter size and reproductive effort within and between some populations of Lacerta vivipara. Holarctic Ecology, 6, 381–386.
Pincheira-Donoso, D., Hodgson, D. J., & Tregenza, T. (2008). The evolution of body size under environmental gradients in ectotherms: Why should Bergmann’s rule apply to lizards? BMC Evolutionary Biolology, 8, 68. doi:10.1186/1471-2148-8-68.
Qualls, C. P., & Shine, R. (1995). Maternal body-volume as a constraint on reproductive output in lizards: Evidence from the evolution of viviparity. Oecologia, 103, 73–78.
Qualls, F. J., & Shine, R. (2000). Post-hatching environment contributes greatly to phenotypic variation between two populations of the Australian garden skink, Lampropholis guichenoti. Biolological Journal of the Linnean Society, 71, 315–341.
Reichling, H. (1957). Transpiration und Vorzugstemperatur Mitteleuropäischer Reptilien und Amphibien. Zoologisches Jahrbuch für Physiologie, 67, 1–64.
Revell, L. J., Harmon, L. J., Langerhans, L. B., & Kolbe, J. J. (2007). A phylogenetic approach to determining the importance of constraint on phenotypic evolution in the neotropical lizard Anolis cristatellus. Evolutionary Ecology Research, 9, 261–282.
Rocha, C. F. D., Vrcibradic, D., Teixeira, R. L., & Cuzzuol, M. G. T. (2002). Interpopulational variation in litter size of the skink Mabuya agilis in southeastern Brazil. Copeia, 2002, 857–864.
Rodríguez-Díaz, T., & Braña, F. (2012). Altitudinal variation in egg retention and rates of embryonic development in oviparous Zootoca vivipara fits predictions from the cold-climate model on the evolution of viviparity. Journal of Evolutionary Biology, 25, 1877–1887.
Roff, D. A. (1992). The evolution of life histories: Theory and analysis. New York: Chapman & Hall.
Rohr, D. H. (1997). Demographic and life-history variation in two proximate populations of viviparous skink separated by a steep altitudinal gradient. Journal of Animal Ecology, 66, 567–578.
Roitberg, E. S., Orlova, V. F., Kuranova, V. N., Bulakhova, N. A., Zinenko, O. I., Ljubisavljević, K., et al. (2011). Inter-observer and intra-observer differences in measuring body length: A test in the common lizard, Zootoca vivipara. Amphibia-Reptilia, 32, 477–484.
Rúa, M., & Galán, P. (2003). Reproductive characteristics of a lowland population of an alpine lizard: Lacerta monticola (Squamata, Lacertidae) in north-west Spain. Animal Biology, 53, 347–366.
Sears, M. W., & Angilletta, M. J. (2004). Body size clines in Sceloporus lizards: Proximate mechanisms and demographic constraints. Integrative and Comparative Biology, 44, 433–442.
Shine, R. (1987). The evolution of viviparity: Ecological correlates of reproductive mode within a genus of Australian snakes (Pseudechis: Elapidae). Copeia, 1987, 551–563.
Shine, R. (1992). Relative clutch mass and body shape in lizards and snakes: Is reproductive investment constrained or optimized? Evolution, 46, 828–833.
Shine, R. (2005). Life-history evolution in reptiles. Annual Review of Ecology Evolution and Systematics, 36, 23–46.
Sinervo, B. (1990). The evolution of maternal investment in lizards: An experimental and comparative analysis of egg size and its effects on offspring performance. Evolution, 44, 279–294.
Smith, R. J. (1999). Statistics of sexual size dimorphism. Journal of Human Evolution, 36, 423–459.
Smith, C. C., & Fretwell, S. D. (1974). The optimal balance between size and number of offspring. American Naturalist, 108, 499–506.
Smith, S. A., & Shine, R. (1997). Intraspecific variation in reproductive mode within the scincid lizard Saiphos equalis. Australian Journal of Zoology, 45, 435–445.
Sorci, G., & Clobert, J. (1999). Natural selection on hatchling body size and mass in two environments in the common lizard (Lacerta vivipara). Evolutionary Ecology Research, 1, 303–316.
Sorci, G., Clobert, J., & Belichon, S. (1996). Phenotypic plasticity of growth and survival in the common lizard Lacerta vivipara. Journal of Animal Ecology, 65, 781–790.
Stearns, S. C. (1992). The evolution of life histories. Oxford: Oxford University Press.
Stone, G. N., Nee, S., & Felsenstein, J. (2011). Controlling for non-independence in comparative analysis of patterns across populations within species. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1569), 1410–1424.
Stuart-Smith, J., Swain, R., Stuart-Smith, R. D., & Wapstra, E. (2007). Is fecundity the ultimate cause of female-biased size dimorphism in a dragon lizard? Journal of Zoology, 273, 266–272.
Sun, Y. Y., Du, Y., Yang, J., Fu, T. B., Lin, C. X., & Ji, X. (2012). Is the evolution of viviparity accompanied by a relative increase in maternal abdomen size in lizards? Evolutionary Biology, 39, 388–399.
Surget-Groba, Y., Heulin, B., Guillaume, C. P., Puky, M., Semenov, D., Orlova, V., et al. (2006). Multiple origins of viviparity, or reversal from viviparity to oviparity? The European common lizard (Zootoca vivipara, Lacertidae) and the evolution of parity. Biological Journal of the Linnean Society, 87, 1–11.
Surget-Groba, Y., Heulin, B., Guillaume, C. P., Thorpe, R. S., Kupriyanova, L., Vogrin, N., et al. (2001). Intraspecific phylogeography of Lacerta vivipara and the evolution of viviparity. Molecular Phylogenetics and Evolution, 18, 449–459.
Taylor, H. L., Cooley, C. R., Aguilar, R. A., & Obana, C. J. (1992). Factors affecting clutch size in the teiid lizards Cnemidophorus tigris gracilis and C. t. septentrionalis. Journal of Herpetology, 26, 443–447.
Tinkle, D. W., Dunham, A. E., & Congdon, J. D. (1993). Life history and demographic variation in the lizard Sceloporus graciosus: A long-term study. Ecology, 74, 2413–2429.
Uller, T., & Olsson, M. (2003). Life in the land of the midnight sun: are northern lizards adapted to longer days? Oikos, 101, 317–322.
Uller, T., & Olsson, M. (2005). Trade-offs between offspring size and number in the lizard Lacerta vivipara: A comparison between field and laboratory conditions. Journal of Zoology London, 265, 295–299.
Van Damme, R., Bauwens, D., & Verheyen, R. F. (1989). Effect of relative clutch mass on sprint speed in the lizard Lacerta vivipara. Journal of Herpetology, 23, 459–461.
Vercken, E., & Clobert, J. (2008). The role of colour polymorphism in social encounters among female common lizards. Herpetological Journal, 18, 223–230.
Vervust, B., Van Dongen, S., & Van Damme, R. (2009). The effect of preservation on lizard morphometrics—An experimental study. Amphibia-Reptilia, 30, 321–329.
Vitt, L. J., & Breitenbach, G. L. (1993). Life histories and reproductive tactics among lizards in the genus Cnemidophorus (Sauria: Teiidae). In J. W. Wright & L. J. Vitt (Eds.), Biology of Whiptail lizards (genus Cnemidophorus) (pp. 211–243). Norman: Oklahoma Museum of Natural History.
Vitt, L. J., & Pianka, E. R. (1994). Lizard ecology: Historical and experimental perspectives. Princeton: Princeton University Press.
Wapstra, E., & Swain, R. (2001). Geographic and annual variation in life-history traits in a temperate zone Australian skink. Journal of Herpetology, 35, 194–203.
Winkler, D. W., & Wallin, K. (1987). Offspring size and number: A life history model linking effort per offspring and total effort. American Naturalist, 129, 708–720.
Yang, J., Sun, Y. Y., Fu, T. B., Xu, D. D., & Ji, X. (2012). Selection for increased maternal body-volume does not differ between two Scincella lizards with different reproductive modes. Zoology, 115, 199–206.
Acknowledgments
We are grateful to Andrey Reshetnikov for profound help with developing Fig. 1, Dennis Rödder for extracting climatic data, and Werner Mayer for correcting the clade assignment of sample 7. We also thank Boris Krasnov, David Tarkhnishvili, and three anonymous reviewers for their comments which resulted in substantial improvement of the quality of the manuscript. This study is part of a larger project supported by the Deutsche Forschungsgemeinschaft (Grant RO 4168/1–1 to ESR).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 6.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Roitberg, E.S., Kuranova, V.N., Bulakhova, N.A. et al. Variation of Reproductive Traits and Female Body Size in the Most Widely-Ranging Terrestrial Reptile: Testing the Effects of Reproductive Mode, Lineage, and Climate. Evol Biol 40, 420–438 (2013). https://doi.org/10.1007/s11692-013-9247-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-013-9247-2