Abstract
The studies of soldered joints were carried out in systems: Al/solder/Al, Al/solder/Cu, Cu/solder/Cu, where the solder was (Al-Zn)EUT, (Al-Zn)EUT with 0.5, 1.0, and 1.5 at.% of Ag and (Al-Zn)EUT with 0.5, 1.0, and 1.5 at.% of Cu addition. Brazing was performed at 500 °C for 3 min. The EDS analysis indicated that the composition of the layers starting from the Cu pad was CuZn, Cu5Zn8, and CuZn4, respectively. Wetting tests were performed at 500 °C for 3, 8, 15, and 30 min, respectively. Thickness of the layers and their kinetics of growth were measured based on the SEM micrographs. The formation of interlayers was not observed from the side of Al pads. On the contrary, dissolution of the Al substrate and migration of Al-rich particles into the bulk of the solder were observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminium and its alloys are one of the main of constructional materials. The use of aluminum, particularly in the automotive industry, is growing rapidly (Ref 1, 2). There is also increasing need for new methods of brazing of aluminum with other materials such as copper. Soldering aluminum with copper and its alloys is widely used in industrial refrigeration, air-conditioning, and extensively applied in heat exchangers. In addition, because of increasing demand for high-temperature solders for brazing in electronics, automotive industry, the Al-Zn alloys with alloying additions seem interesting proposition (Ref 3). For the development of new technologies and design of new materials, it is important to know the processes taking place at the interface of connected elements with solder (Ref 4). Taking into account, the Directives of the European Parliament and the Council of Europe adopted in 2003 (Ref 5-8) forbade to use solders containing metals which are hazardous to health—lead and cadmium. This forced manufacturers to use, and research institutes to search for, new ecological alloys. In the case of low-melting solders, the studies are mainly focused on the eutectic Sn-Ag, SnAgCu, and SnZn with Ag, In, Bi, Al additions (Ref 9, 10) and for solders with melting points above 350 °C on eutectic ZnAl with Ag, Cu, In, Sn additions (Ref 11-13). In the literature, more attention is paid to analysis of the microstructure of solder joints and the influence of various alloying elements on the microstructure (Ref 11-15). The studies usually concern the kinetics of IMCs growth on the substrate and the influence of IMCs on the properties and aging of the joints.
Some of the most frequently analyzed solders are these based on eutectic Zn-Al, as they are characterized by low cost, low electrical resistance, good mechanical properties (Ref 13) and twice as high hardness and thermal conductivity comparing to lead-based solders (Ref 15). These properties allow to use the eutectic Al-Zn for brazing not only copper but also aluminum, nickel, or steel. What is especially interesting, the solder offers the possibility to solder two different metals—aluminum and copper. However, the presence of water or water vapor at the Cu-Al interface causes the formation of corrosion cells (Ref 16). In addition, copper and aluminum show different electrical resistance and thermal expansion. Therefore, it is crucial to use proper coatings for brazing or use alloys which form buffer layers during the process of brazing of copper with aluminum. Considering the above, it seems interesting to analyze the processes at the substrate/solder/substrate interfaces. The literature describing the phenomena occurring at the interface of liquid solder (Al-Zn eutectic and Al-Zn eutectic + Cu)/Cu pad (Ref 11-13) or Al-Zn eutectic/Cu or Al substrate (Ref 17) indicates that the processes taking place at the interface between the liquid solder/Cu substrate correspond to rich in intermetallic phases Cu-Zn system. The authors stated the formation of the intermediate layers composed of three intermetallic phases: β-CuZn, ε-CuZn4, and γ-Cu5Zn8 at the solder/Cu interface. It seems that the fast growth of phases can cause cracks in the joints and poor connection effect in the case of brazing copper with other metals. The authors (Ref 18) studied the kinetics of intermetallic phases’ formation at the interface of solder (AlZnAg)/substrate (Cu) after a short wetting time. They observed that even after 3 s of annealing, the formation of the three phases occurs. Moreover, they suggest that the addition of Ag to the alloy reduces the rate of the formation of brittle β and γ phases.
This paper presents a description of the phenomena that occur at the interface Al/solder/Al, Al/solder/Cu, Cu/solder Cu and may be helpful in the design of abovementioned solder joints/systems.
Experimental
The alloys for tests were prepared by melting pure metals (Zn 99.95%, Al—99.99%, Ag—99.999%, Cu—99.99%) in graphite crucibles, in a glovebox filled with Ar (99.9992%) protective atmosphere (oxygen and water vapor pressure below 1 ppm) to avoid oxidation. The solder compositions are shown in Table 1.
The solders after casting were rolled to a thickness of 0.25 mm. Brazing tests were carried out on copper (99.95%) and aluminum pads (99.9%). Copper pads were prepared in accordance with EN ISO 9455-10:2000, and aluminum pads after pre-treatment of oxides were degreased with acetone, and polishing was performed on “Struers LectroPol 5.” The pads were in a rectangular shape with dimensions of 25 × 10 × 4 mm. Brazing was performed in a furnace as described in Ref 17 under a protective atmosphere of nitrogen using ALU33® flux. Wetting tests were performed at 500 °C for 3, 8, 15, and 30 min, respectively. The process of brazing was held in the holder as shown in Fig. 1 with different clamping forces of the springs applied. The force was regulated with screws what allowed the authors to control the amount of solder reacting at the joint. After brazing tests, the solder connection was investigated of the microstructure. The microstructural and EDS analysis was performed with Quanta 3D FEG system, at 20 kV, with the use of the standardless Analysis EDAX System based on Genesis 4000 software, while phase composition was studied with x-ray diffraction (XRD). For all samples, three measurements were made at different areas to improve counting statistics and to check homogeneity of joints. First XRD measurement was performed in the center of joint, and on opposite edges, measurements were supposed to help to determine the best of characteristic of materials. In all figures and text, compositions are given in at.%.
Results and Discussion
The brazing was performed at 500 °C for 3 min in the case of the Al pads and for 3, 8, 15, and 30 min in the case of the Cu pads. The example microstructures of the obtained joints are shown in Fig. 2, 3, 4, 5, 6, and 7.
Al/solder/Al
The microstructures presented in Fig. 2 and 3 show the reaction occurring at the interface between the liquid solder and the Al pads, described in Ref 19, 20. That is a cyclic mechanism of dissolution and crystallization. The liquid solder dissolves the Al pads, and the resulting alloy crystallizes forming so called “scallops,” separated from each other by channels with liquid solder. The liquid solder in the channels causes further dissolution, and the alloy formed in this way yields crystallization. Despite the fact that the Zn-Al eutectic alloy with 12 at.% of aluminum and small additions of silver or copper was used as the solder, the joint contains mainly aluminum from the dissolved Al pads, especially in the case when the joints were prepared with a strong force (Fig. 3). Indirectly, this confirms the existence of the mechanism described in paper (Ref 20). The authors (Ref 20) studying the processes occurring at the interface between the liquid aluminum and the nickel pad noticed that there are three zones: d x —dissolution zone, s r—saturation zone, and s s—supersaturation zone. In the first one, the liquid dissolves the pad, until the product of this reaction becomes a liquid having the N 0 composition. Then the liquid diffuses and crystallizes. This zone is difficult to find as it is very small and does not exists for a long time. On the microstructures shown in Fig. 2b and 3a, one can extract the two other zones—s r and s s which differ in the content of dissolved zinc. Silver and copper which are added to the solder concentrate on the edge of “scallops,” and they are dissolved in the Al40Zn60 phase. Subsequently, it can be assumed that they react with the liquid zinc resulting from the previous reaction and form small grains of intermetallic compounds (IMCs): Cu5Zn8. The stronger pressure caused the decrease of the solder amount in the joint and hence a much larger part of the solder reacted with the pads forming three times larger structures. Moreover, in some cases (Fig. 3a), the formation of the delamination process was observed. The reaction products of the two Al pads were separated by the unreacted eutectic solder.
Al/solder/Cu
Figure 4 and 5 illustrate the microstructures of Al/solder/Cu joints prepared with the high-pressure (Fig. 4) and with the low-pressure (Fig. 5) springs. Similarly as before, the existence of s r and s s zones was noticed at the aluminum pad side. From the copper pad side, one can observe the creation of the intermediate layer consisting of the three intermetallic phases in the following sequence (starting from copper): β-Cu[Zn,Al] γ-Cu5[Zn,Al]8, and ε-Cu[Zn,Al]4. It can be presumed that the above-described mechanism of dissolution and crystallization occurs (Ref 20). Cu and Ag additions cause the emergence of precipitates rich in Ag or Cu and the propagation of these elements to the eutectic. Moreover, the silver is dissolved in the Al40Zn60 phase. Higher pressure slows the rate of the phases’ growth. This is probably related to the increase of aluminum concentration which dissolved from the pads to the solder.
Cu/solder/Cu
The Gibbs phase rule states that during the process of crystallization at the constant temperature of 500 °C in the ternary (Al, Zn, Cu or Ag) system, there are four phases in the equilibrium state. As previously mentioned, the intermediate layer composed of three intermetallic phases with different crystal lattices: β-Cu[Zn,Al], γ-Cu5[Zn,Al]8, and ε-Cu[Zn,Al]4 forms at the solder-Cu pad interface. The fourth phase is the supercooled liquid (d x ) with the N 0 composition. The composition of N 0 was calculated from the mass balance equation (1).
where d 1 , d 2 , d 3 are the thickness of the phases formed in one joint after a specific brazing time and N Cu i is the average copper content in the “i” phase.
The calculations were carried out for all the solders after each brazing time and for different contact forces. The results showed that the copper content ranged from 28% for solder with a high content of alloying elements and the shortest time of brazing, to 35% for the pure eutectic after the longest period of time. It seems that the longer wetting time, the more important role that the phenomenon of diffusion played, and hence the difference of about 7% Cu occurred. The average value of N 0 for all the measurements amounted to 31% Cu.
From the microstructures shown in Fig. 4, 5, 6, 7, and 8, one can conclude that from the liquid N 0 composition, the intermetallic phases crystallize with the following sequence: β-Cu[Zn,Al], γ-Cu5[Zn,Al]8, and ε-Cu[Zn,Al]4. The Al-Zn-Cu phase diagram (Ref 21) shows that the excessive amount of zinc (in relation to the calculated from the mass balance) is needed to keep the dissolution zone (d x ) liquid. During the crystallization process, the intermetallic layer of the total N 0 composition is formed, and the excess of zinc returns to the solder. The areas with high concentration of zinc can be seen on the microstructures shown in Fig. 8. According to (Ref 21) aluminum replaces zinc in the intermetallic phases, and the process is the easiest in the γ phase. Silver is most easily dissolved in the ε phase (Fig. 6).
Occurrence of IMPs layers was improved by XRDs measurements presented in Fig. 9(a) ZnAl + 1.5Ag and (b) ZnAl + 1.5Cu, respectively, in both case for connections Cu/solder/Cu. Measurements were conducted for three area of interactions pads with solder, at the edges and in the middle of connections. In both cases, the XRDs results show occurrence of IMPs, γ-Cu5[Zn,Al]8, ε-Cu[Zn,Al]4, and for alloys ZnAl with Ag the AgZn3. As is apparent from phase diagram (Ref 21), and XRDs measurements in the γ phase dissolution Al were observed.
The dependence of the change of thickness of the phase over time can be described by the following relationship:
where δ is the average thickness of the reaction layer at time t, k is the growth rate constant, and n is the time exponent.
Generally, the obtained value of n determines the types of layer growth at the interface and the possible reaction mechanisms. Takaku et al. (Ref 12) suggested that for reaction of Al-Zn and Al-Zn-1%Cu solders with Cu pads, the exponent n is 0.5, which means that growth of phase is controlled by the volume diffusion. Table 2 shows the values of the coefficient k calculated under the assumption that n = 0.5. These data relate to solders with different contents of Cu and Ag, and connections obtained by different degrees of pressure. These data indicate that the greatest rate of increase has phase γ and the lowest β. Additives Cu to eutectic Al-Zn cause an increase in the rate of formation phase γ and ε, the greater share of Cu the faster growth of phases, while the Ag additives generally do not change the k value. The rate of formation of the phases is affected by different degrees of pressure; for the higher pressure, the growth of γ phase will be faster.
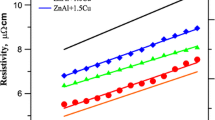
The kinetics of intermetallic phases growth at the liquid solder eutectic ZnAl and ZnAl with 1% Cu—copper pad on Fig. 10, was presented. The obtained results were compared with literature data (Ref 11) interface at low pressure. The kinetics of intermetallic phases’ growth at the liquid solder eutectic ZnAl and ZnAl with 1% Cu-copper pad interface at 500 °C is presented in Fig. 10. The obtained results were compared with literature data (Ref 12). Authors (Ref 12) obtained higher values of the coefficients k for phases, γ and β, and the lower for ε phase.
Kinetics of growth of IMPs vs. square root of time for (a) Zn12Al and (b) Zn12Al + 1.0%Cu. Points—present work, dashed line—(Ref 12) at temperature 500 °C
Analyzing Fig. 10, we can see that the value of the exponent of time n for the phases, ε and β, oscillates around the value of 0.5, which suggests that the growth of these phases is controlled by the volume diffusion mechanism. However, the γ phase values of n differ considerably from 0.5.
Considering the above, in Fig. 11(a-c) and 12(a-c), dependence of thickness of the phases is shown as a function of time, with the calculated coefficients n and k. These coefficients were calculated using the program GRAPHER_6, using Eq 2. The results of calculations shown in Fig. 11 and 12 show that the exponent of time n for phase γ in the solders of different contents of Ag and Cu ranges from 0.86 to 0.9. This suggests that the growth of γ phase is controlled not only on the volume diffusion but also on diffusion of the grain boundaries. This may result from the fact that this phase has the lowest activation energy and the lowest Gibbs’ free energy (Ref 13, 17). Figure 13 shows a comparison of the kinetics of the phases’ growth with high and low pressure applied. In the case of low pressure, there was a large amount of liquid solder to react. This resulted in a higher rate of ε phase growth (Fig. 13a) and did not cause the inhomogeneity of Zn concentration (Fig. 7). However, in the case of high-pressure application, the regions rich in zinc appeared, and the growth of the γ phase was faster. This can be probably related to the fact that in this case, the diffusion processes were responsible for the growth of the phase.
Conclusions
It was observed that during the wetting of the Cu and Al substrates with (Al-Zn)eut solder with Ag and Cu additions at T = 500 °C, the cyclical process of dissolution and crystallization described in Ref 19, 20 occurs. The liquid solder dissolves the aluminum pad and causes the diffusion of aluminum to the solder. The grain structure is formed, and the particle size is dependent on the amount of the solder (the value of the applied pressure) and on the alloying additions. The higher pressure causes the coarsening of the grains. The additions of silver and copper to the solder concentrate on the edge of “scallops,” and then, they are dissolved in the Al40Zn60 phase.
In the Cu/solder/Al joints, the additions of Cu and Ag cause the emergence of precipitates rich in Ag or Cu and also the diffusion of these elements to the eutectic. Furthermore, from the Al side, the Ag is dissolved in Al40Zn60 phase. The higher pressure causes the decrease of the rate of the phases’ growth.
The liquid solder in the interaction with the Cu substrate forms a layer of three IMCs: β-CuZn, ε-CuZn4, and γ-Cu5Zn8. These phases are formed as the result of dissolution and crystallization mechanisms and the phenomenon of diffusion. The Al from the solder diffuses to the γ phase, substituting Zn, according to the phase diagram (Ref 21). However, in the ε phase, the Ag is dissolved. Furthermore, the addition of Cu to the eutectic Zn-Al causes the inhibition of the brittle β phase growth. In the case of the application of low pressure, the rate of the ε phase growth was higher. The γ phase grew faster in the case of high pressure used.
Global demand for materials of specific properties is increasing, including braze materials. In the process of design and development of braze materials tests of wettability, microstructural and mechanical properties of joints are needed. Phenomena occurring of substrate-solder interface are also interesting. Because of e difference in thermal expansion, coefficient of Cu and Al braze needs to compensate it. The result of present paper is useful for design of brazed joints of the types: Cu/braze/Cu, Al/braze/Cu, and Al/braze/Al.
The exponent of time n for the phases, ε and β, oscillates around the value of 0.5, which suggests that the growth of these phases is controlled by the volume diffusion mechanism. For γ phase, exponent of time n ranges from 0.86 to 0.9. This suggests that the growth of γ phase is controlled not only by on the volume diffusion but also by on diffusion along the grain boundaries.
References
K. Sears, Automotive Engineering: Strategic Overview, Steel World, 1997, 2(1), p 55–68
W.S. Miller, L. Zhuang, J. Bottema, A.J. Wittebrood, P. De Smet, A. Haszler, and A. Vieregge, Recent Development in Aluminium Alloys for the Automotive Industry, Mater. Sci. Eng., 2000, A280, p 37–49
R.B. Brown, F.L. Terry, and K. Wu, High-Temperature Electronics, R.K. Krischman, Ed., IEEE Press, New York, 1999, p 223–226
H.H. Manko, Solders and Soldering, 4th ed., McGraw-Hill, New York, 2001
Directive 2002/96/EC of the European Parliament and of the Council, http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:037:0024:0038:en:PDF. Accessed 17 Mar 2014
Directive 2003/108/EC of the European Parliament and of the Council, http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:345:0106:0107:EN:PDF. Accessed 17 Mar 2014
Directive 2008/35/EC of the European Parliament and of the Council, http://www.rreuse.org/t3/fileadmin/editor-mount/documents/100/00179-ROH-Samend.pdf. Accessed 17 Mar 2014
E.P. Lopez, P.T. Vianco, and J.A. Rejent, Solderability Testing of Sn-Ag-XCu Pb-Free Solders on Copper and Au-Ni Plated Kovar Substrates, J. Electron. Mater., 2005, 34, p 299–310
Z. Moser, W. Gąsior, J. Pstruś, and A. Dembski, Wettability Studies of Pb-Free Brazing Materials, Int. J. Thermophys., 2008, 29, p 1974–1986
H.Y. Xu, Z.F. Yuan, H. Matsuura, and F. Tsukihashi, Hysteresis Phenomenon and Wetting Characteristics of Molten Sn-3.0 wt.%Ag-0.5 wt.%Cu on different tilting substrates, J. Alloys Compd., 2009, 487(1–2), p 121–125
T. Savaskan and M.S. Turhal, Reationships Between Cooling Rate, Copper Content and Mechanical Properties of Monotectoid Based Zn-Al-Cu Alloys, Mater. Charact., 2003, 51, p 258–270
Y. Takaku, L. Felicia, I. Ohnuma, R. Kainuma, and K. Ishida, Interfacial Reaction Between Cu Substrates and Zn-Al Based High-Temperature Pb-Free Solders, J. Electron. Mater., 2008, 37, p 314–323
N. Kang, H.S. Na, S.J. Kim, and C.Y. Kang, Alloy Design of Zn-Al-Cu Solder for Ultra High Temperatures, J. Alloys Compd., 2009, 467, p 246–250
P. Sebo, Z. Moser, P. Svec, D. Janickovic, E. Dobrocka, W. Gąsior, and J. Pstruś, Effect of Indium on the Microstructure of the Interface Between Sn3.13Ag0.74CuIn Solder and Cu Substrate, J. Alloys Compd., 2009, 480, p 409–415
T. Shimizu, H. Ishikawa, I. Ohnuma, and K. Ishida, Zn-Al-Mg-Ga Alloys as Pb-Free Solder for Die-Attaching Use, J. Electron. Mater., 1999, 28(11), p 1172–1175
H.H. Manko, Solders and Brazing, McGraw-Hill, New York, 2001
J. Pstruś, P. Fima, and T. Gancarz, Wetting of Cu and Al by Sn-Zn and Zn-Al Eutectic Alloys, J. Mater. Eng. Perform., 2012, 5(21), p 606–613
T. Gancarz, J. Pstruś, P. Fima, and S. Mosinska, Effect of Ag Addition to Zn-12Al Alloy on Kinetics of Growth of Intermediate Phases on Cu Substrate, J. Alloys Compd., 2013, 582, p 313–332
W. Wołczyński, J. Janczak-Rusch, J. Kloch, T. Rutti, and T. Okane, A Model for Solidification of Intermetallic Phases from Ni-Al System and Its Application to Diffusion Brazing, Arch. Metall. Mater., 2005, 50, p 1055–1068
W. Wołczyński, T. Okane, C. Senderowski, B. Kania, D. Zasada, and J. Janczak-Rusch, Meta-stable Conditions for Diffusion Brazing, Arch. Metall. Mater., 2011, 56, p 311–323
V. Raghavan, Al-Cu-Zn (Aluminum-Copper-Zinc), J. Phase Equilib. Diffus., 2007, 28(2), p 183–188
Acknowledgments
This work was financed under the framework of the Project POIG.01.01.02-00-015/09, co-funded by the European Regional Development Fund (ERDF) and the Government of Poland under the Innovative Economy Program in the years 2010-2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is an invited submission to JMEP selected from presentations at the Symposia “Wetting,” “Interface Design,” and “Joining Technologies” belonging to the Topic “Joining and Interface Design” at the European Congress and Exhibition on Advanced Materials and Processes (EUROMAT 2013), held September 8-13, 2013, in Sevilla, Spain, and has been expanded from the original presentation.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Pstruś, J., Gancarz, T. Interfacial Phenomena in Al/Al, Al/Cu, and Cu/Cu Joints Soldered Using an Al-Zn Alloy with Ag or Cu Additions. J. of Materi Eng and Perform 23, 1614–1624 (2014). https://doi.org/10.1007/s11665-014-0942-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-014-0942-7